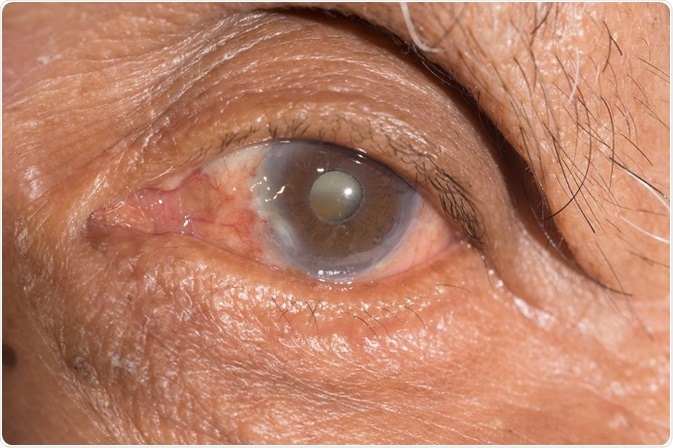
Cataract is a clouding of the lens within the eye and affects tens of millions of people. At present, there is no cure for cataract, and consequently the only available method to treat cataract is by surgical removal.
Credit: ARZTSAMUI/ Shutterstock.com
By the year 2020, it is estimated that >30 million operations to remove cataract will be performed annually and these numbers are likely to grow in the future as lifespan and consequently the ageing populous increase.
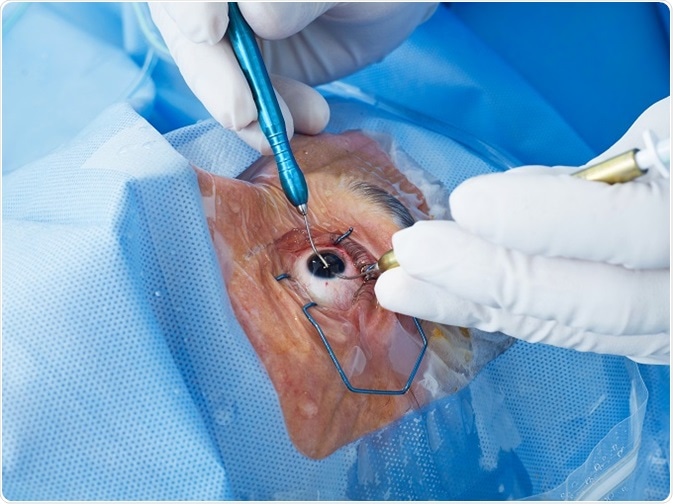
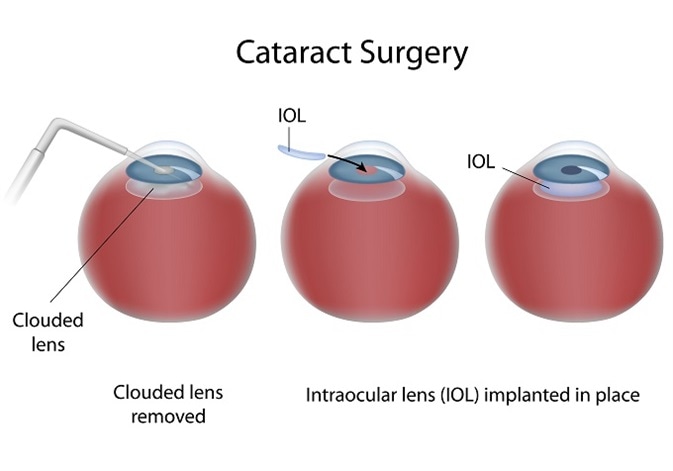
Cataract surgery is initially successful and involves creating a circular hole in the front part of the lens (the anterior capsule) to allow access to the central lens material (fibre cells), which is typically the site of the cataract. This central lens material is then removed.
Credit: goir / Shutterstock.com
Following this procedure, a ring of anterior and the entire posterior lens capsule remain. This is known as the capsular bag and in which an artificial intraocular lens (IOL) can be implanted. Having removed the clouded component of the lens, light can pass through the visual axis uninterrupted and with the aid of the IOL the patient can focus on the retina.
Credit: Alila Medical Media / Shutterstock.com
Unfortunately, surgery provokes lens epithelial cells attached to the capsule to undergo a wound healing response in a significant proportion of patients. This causes light scatter in the visual axis, consequently reducing visual quality.
This condition is known as Posterior capsule opacification (PCO) and requires laser surgery to correct; this is the second most common operative procedure in the world. Reducing the impact of PCO on patients is of great importance and novel approaches need to be developed.
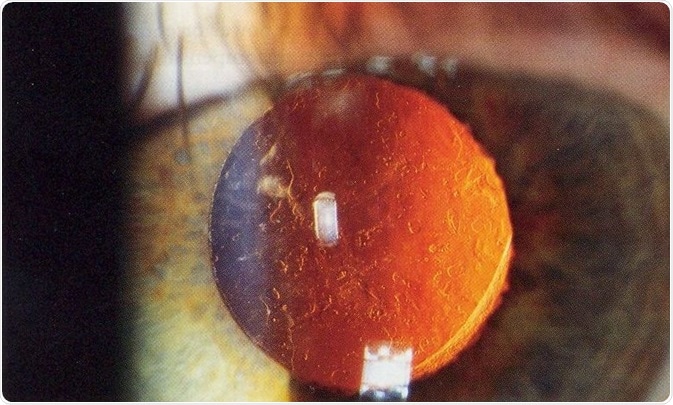
After cataract - Posterior capsular opacification post-cataract surgery (seen on retroillumination). Credit: Rakesh Ahuja, MD.
Basic biology behind PCO
Considerable work has taken place to better understand the underpinning biology driving PCO. This in turn will help develop novel targets for future therapies and facilitate improvement in IOL designs. PCO is characterized by a number of biological events.
Fundamentally all PCO exhibits some level of fibrosis, which is characterized by increased cell numbers, cell migration, a change in cell type from an epithelial cell to a myofibroblast (these are typically associated with injury responses), deposition of matrix material (e.g. collagen) and deformation of the matrix (lens capsule).
In addition, attempts to regenerate the lens are evident and formation of new lens fiber cells and fiber-like cells called Elschnig’s pearls can also be observed, which can also cause considerable light scatter.
A number of growth factors and signaling pathways have been identified in association with many of the characteristic events seen in PCO. An incredible feature of the lens is that it does not receive a blood supply and actually lives in a relatively poor environment. In response to these conditions the lens has an innate capacity to generate molecules that govern lens cell functions.
Following cataract surgery, lens cells retain this ability and thus factors generated in response to injury can activate corresponding receptors and contribute to PCO formation through a self-regulated (autocrine) mechanism.
Molecules believed to play a role in this process include fibroblast growth factor (FGF) and vascular endothelial growth factor (VEGF). PCO is also influenced by changes to the eye resulting from surgery. In the days and weeks following a cataract operation levels of protein in the fluids that bathe the lens are increased due to production by ocular tissues and introduction of proteins from the blood.
One protein that demonstrates increased levels is transforming growth factor beta (TGFβ). This is a molecule commonly associated with fibrosis throughout the body and evidence supports a role for TGFβ in PCO.
Interestingly a short exposure of TGFβ to the capsular bag can result in long-term changes. This appears to result from TGFβ binding to the capsule, which provides a reservoir of the molecule, which is presented to the growing lens cells for extended periods.
In essence, events immediately following surgery lay down a platform that will progress PCO to a certain degree, but persistent ongoing processes over months and years ultimately give rise to clinically significant PCO.
Pharmacological approaches
Better understanding of the biology of PCO has identified different therapeutic strategies to prevent it. These approaches aim to disrupt specific events that contribute to the condition. Some of the agents proposed have aimed to reduce proliferation and/or migration of lens epithelial cells with the belief that if the cells to not reach the visual axis minimal visual disruption will occur.
Other strategies have been directed to reducing myofibroblast formation, which is also believed to promote matrix deformation; many of these approaches have focused on disrupting TGFβ signaling. The principle here is that a single layer of cells within the visual axis will not cause light scatter and retention of cells will stabilize the IOL within the capsular bag.
Alternatively, complete annihilation of lens cells within the capsular bag also remains an option. In all cases in which a therapeutic agent is to be employed it is vital that the delivery is controlled to the target cells i.e. lens cells and that other ocular tissues are not affected.
Attempts to use pharmacological agents clinically to prevent PCO have been limited, but as more focused approaches are developed, and drug delivery improves, this remains a possibility in the future.
Intraocular lenses
IOLs appear to be the best tool currently available to manage PCO. There are a number of designs established from a variety of materials, but in this instance I will highlight three design concepts.
Square-edge IOLs
A number of IOLs are now manufactured to ensure the edge of the optic component exhibits a sharp edge. The underlying principle is that the lens posterior capsule is stretched over the sharp edge forming a tight interaction.
Following surgery, typically the front surface of the lens will stick to the back surface of the lens and the IOL. This contact results from cell-cell interactions and release of biological materials, which act like glues fusing the different parts together. This scenario can be described as a closed-capsular bag system.
Clinical research shows that a square-edge profile to the IOL inhibits LEC movement onto the back of the lens and reduces PCO. This design is currently the most commonly employed, but it has become apparent that this only delays rather than cures the problem.
Open-bag IOLs
An alternative approach to closed-bag IOLs has now been proposed that aims to introduce open-bag devices. In this case the capsular bag is kept open with separation of contact between the front and back surfaces of the lens.
Recent experimental and clinical observations, suggest that if the lens capsular bag can be held open after surgery so that the front and back surfaces are separate and aqueous humour can flow in and out of the capsular bag, the dynamics of lens cell biology are profoundly altered.
There is now excellent experimental and clinical evidence showing that this leads to improved management of PCO. It is therefore likely that IOL designs utilising the open-bag principle will be implanted in higher numbers in the future.
The bag-in-the-lens IOL
In contrast to conventional surgery, which requires a single opening in the anterior capsule, the bag-in-the-lens technique requires making an opening of the same size in the anterior and posterior lens capsules. The edges of the capsule openings are then slotted into a groove at the periphery of the IOL in a similar manner to a tyre being introduced to a wheel.
According to this concept, if both capsules are placed in the groove and are well stretched around the optic of the IOL any lens epithelial cells will be captured within the remaining space of the capsular bag and their growth will be limited to this space, so the visual axis can remain clear.
Significant numbers of BILs have been implanted now and the outcomes are impressive. Of particular interest is the application to children, who have rapid onset of blinding PCO; the BIL IOL prevents PCO, even in these extreme cases.
The limiting factor in the uptake of this approach is the level of skill required by the surgeon to carry out this procedure. However, instructional courses are available along with an international panel of BIL instructors who can pass on their knowledge and demonstrate the technique.
Alternative surgery
Recent work has suggested that creating a small, off-set opening in the anterior capsule and retention of the lens epithelial cells can facilitate regeneration of a new lens from endogenous stem cells within the epithelial cell population.
This work was carried out on infants who presented congenital cataract (present from birth). There was clear evidence that some level of lens regeneration had occurred and provided some benefit to these patients.
However, a number of issues need to be considered when contemplating this approach as an alternative for conventional surgery. The capacity to repair and regenerate is faster in the young and several months were required to see some level of visual improvement. In older patients who make up the majority of cataract patients the ability to reform a lens will be significantly slower and less likely to be successful.
For this new form of surgery to be successful, the regenerated lens will need to closely reflect the normal healthy human lens and do so relatively quickly otherwise further care will be required. Lens regeneration surgery holds great promise, but at present has significant limitations that will prevent mass introduction of this technique for cataract patients.
Summary
Cataract is a major healthcare problem throughout the world and will continue to be as our ageing population expands. Resolution of cataract can only be achieved through cataract surgery and while initially successful, wound-healing responses to surgical injury lead to secondary visual loss in a significant number of patients.
Over the past few decades a greater understanding of the biological basis of PCO has been established and has identified possible therapeutic targets. In addition, IOL designs continue to improve, such that clinically relevant PCO is reduced, but the problem remains.
Further improvements in IOL design are required that could be used in concert with pharmacological agents to further reduce or prevent PCO formation in the future, which would improve the lives of millions.
Acknowledgements

Fight for sight - Someone in the world goes blind every five seconds. Our mission is to stop sight loss in its tracks. By funding pioneering eye research, we’re creating a future everyone can see.
- Moorfields Eye Hospital
- UCL Institute of Ophthalmology
- The Choroideremia Research Foundation
Further Reading
About Dr. Michael Wormstone

Dr Michael Wormstone is a biomedical scientist with primary interests in the use of human tissue to study human eye disease. In particular, he is interested in wound-healing events that follow cataract surgery, which lead to a secondary visual loss known as posterior capsule opacification (PCO).
Through the use of human tissue the laboratory aims to further our understanding of the cell and molecular mechanisms underpinning PCO and identify novel targets for future therapeutic development.
Michael was made a fellow of the Association for Research in Vision and Ophthalmology (ARVO) in 2016, is a visiting Professor of Harbin Medical University (China) and was the 2015 recipient of the National (USA) Foundation for Eye Research Cataract Research Award for outstanding research achievements.
References
- Wormstone, I. M., Wang, L. X. & Liu, C. S. C. (2009) Posterior capsule opacification. Exp Eye Res 88:257-269. doi:10.1016/j.exer.2008.10.016.
- Wormstone IM, Eldred JA (2016) Experimental models for posterior capsule opacification research. Exp Eye Res 142:2-12. doi:10.1016/j.exer.2015.04.021
- Eldred, J. A., Spalton, D. J. & Wormstone, I. M. (2014) An in vitro evaluation of the Anew Zephyr open-bag IOL in the prevention of posterior capsule opacification using a human capsular bag model. Invest Ophthalmol Vis Sci 55:7057-7064. doi:10.1167/iovs.14-15302
- Eldred JA, McDonald M, Wilkes HS, Spalton DJ, Wormstone IM (2016) Growth factor restriction impedes progression of wound healing following cataract surgery: identification of VEGF as a putative therapeutic target. Scientific reports 6:24453. doi:10.1038/srep24453
- Lin H et al. (2016) Lens regeneration using endogenous stem cells with gain of visual function. Nature 531: 323-328. doi:10.1038/nature17181
- MAIDMENT JA, TAMIYA S, COLLISON DJ, WANG L, DUNCAN G AND WORMSTONE IM. Regional Differences in Epidermal Growth Factor Receptor Signaling Components in the Human Lens. Invest Ophthalmol Vis Sci. 2004, 45:1427-35.
- WORMSTONE IM. Posterior Capsule Opacification: A Cell Biological Perspective. Exp. Eye Res. 2002, 74:337-347.
- ELDRED JA, SPALTON DJ, WORMSTONE IM. An in vitro evaluation of the Anew Zephyr® open bag IOL in the prevention of Posterior Capsule Opacification using a human capsular bag model. Invest. Ophthalmol. Vis. Sci. 2014; 55:7057-7064.
Disclaimer: This article has not been subjected to peer review and is presented as the personal views of a qualified expert in the subject in accordance with the general terms and condition of use of the News-Medical.Net website.
Last Updated: Jun 26, 2019