From CN BioReviewed by Olivia Frost
This article is based on a poster originally authored by Yassen Abbas, Hailey Sze, Christiana Skarlatopoulou, Ashley A. Spreen, Elizabeth M. Boazak, William R. Thelin, and Tomasz Kostrzewski which was presented at ELRIG 2024 in affiliation with CN-Bio.
This poster is being hosted on this website in its raw form, without modifications. It has not undergone peer review but has been reviewed to meet AZoNetwork's editorial quality standards. The information contained is for informational purposes only and should not be considered validated by independent peer assessment.

Efforts to improve the in vitro to in vivo translation of drug efficacy and safety data have led to the emergence of more physiologically relevant microphysiological systems (MPS) that consist of multiple fluidically linked organs.1
This study combined two established and well-characterized human MPSs, the RepliGut® Jejunum and PhysioMimix® Liver MPS, in an interconnected dual-organ MPS, to create a Gut/Liver system capable of profiling oral bioavailability.
Defining cell validation criteria is important to ensure donor lots of liver and intestinal cells are functional in co-culture and meet the threshold for regulatory requirements and market adoption.2
This article defines cell validation criteria whereby liver and intestinal cells are first validated separately, then as a functional, fluidically coupled co-culture system.
Using a test compound ensured the cells in co-culture were metabolically suitable for ADME studies, and through real-world drug examples, how the Gut/Liver MPS can be used to provide a mechanistic understanding of a drug’s oral bioavailability in vitro is demonstrated.
Methods
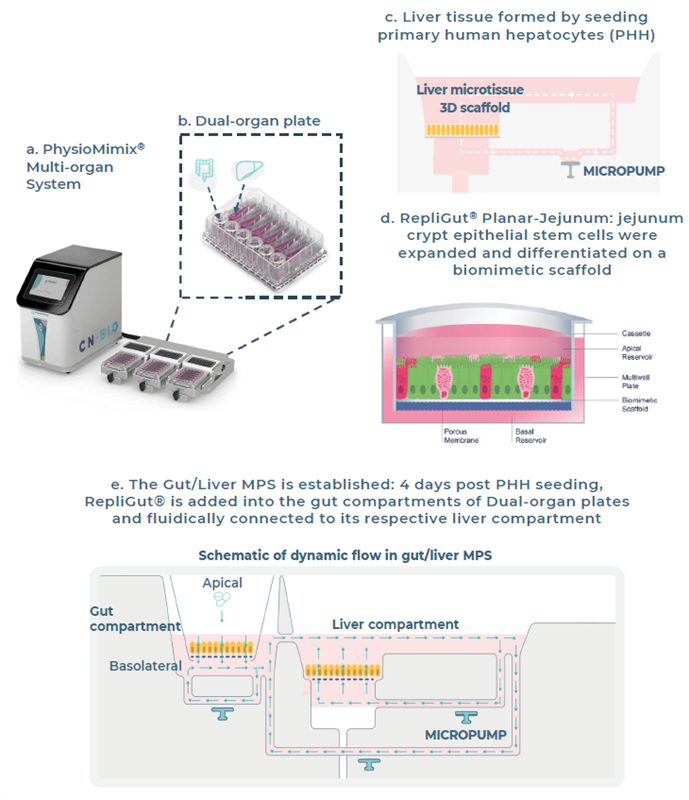
Fig 1. Establishment of the Gut/Liver MPS. Image Credit: Zyme Communications Ltd
Results
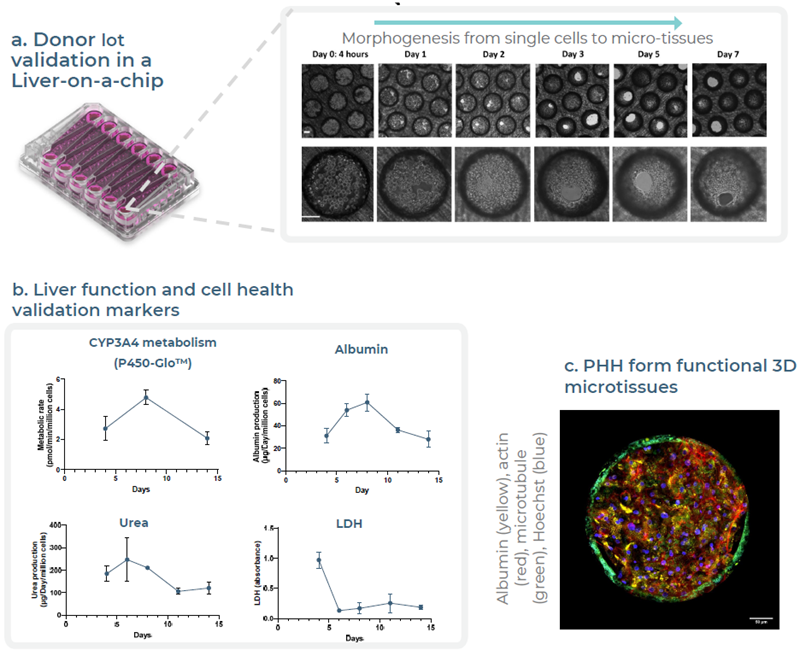
Fig 2. Validation of PHH donor first in a Liver-on-a-chip to confirm metabolically functional tissue. Image Credit: Zyme Communications Ltd
- PHH donors are pre-selected for a Gut/Liver MPS by first validating in a Liver-on-a-chip over a 14-day experiment.
- Functionality is assessed by CYP3A4 activity, albumin, and urea production. Lactate dehydrogenase, a cell health marker, peaks at day 4 after the formation of microtissues and then remains low over the course of the experiment.
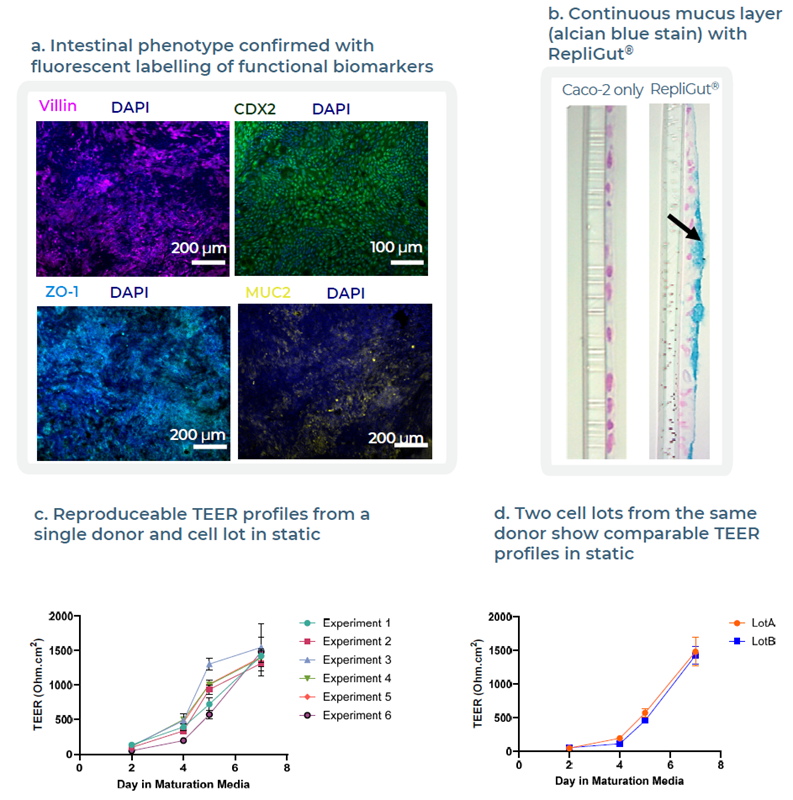
Fig 3. Validation of RepliGut® donor to confirm tissue functionality and reproducibility. Image Credit: Zyme Communications Ltd
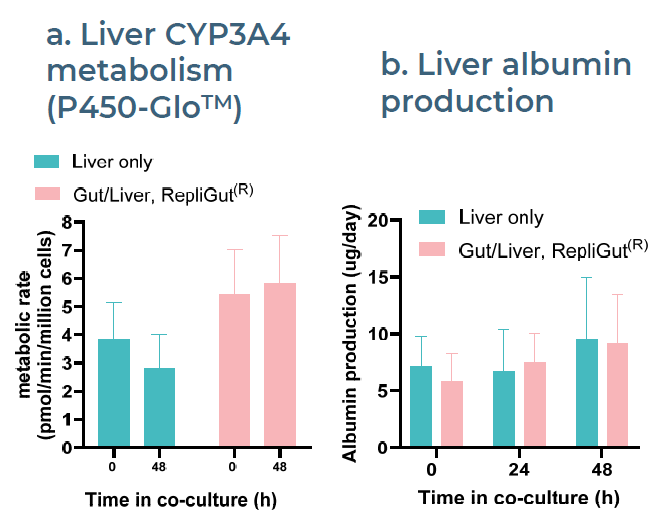
Fig 4. PHH functionality is maintained for at least 48 hours in co-culture with RepliGut®. Image Credit: Zyme Communications Ltd
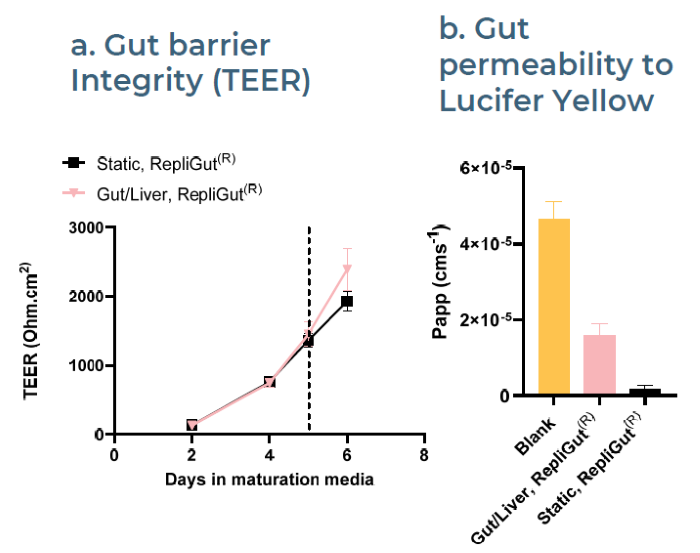
Fig 5. Intestinal barrier is maintained in co-culture with liver microtissues. Image Credit: Zyme Communications Ltd
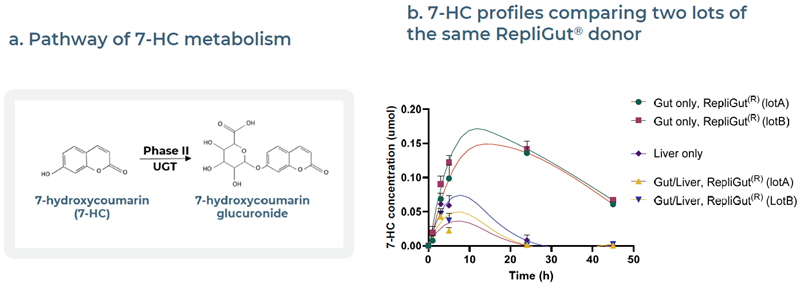
Fig 6. Validation with ADME test compound, 7-hydroxycoumarin (7-HC), to confirm intestinal absorption and hepatic metabolism in gut-only, liver-only, and Gut/Liver MPS models. Image Credit: Zyme Communications Ltd
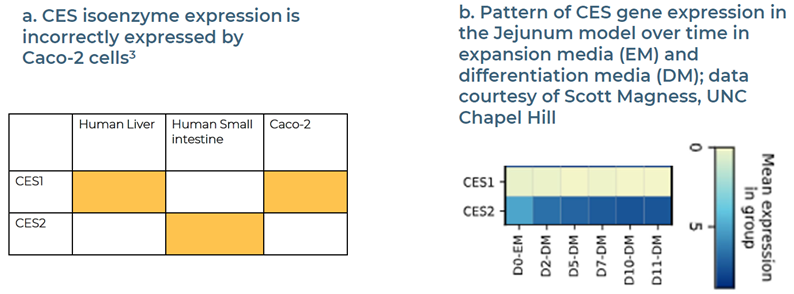
Fig 7. Case studies: profiling bioavailability on carboxylesterase-mediated compounds. Image Credit: Zyme Communications Ltd
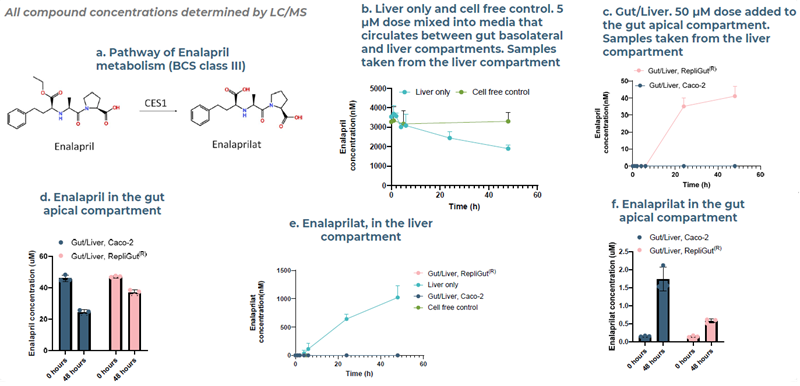
Fig 8. Case study 1, Enalapril: greater resistance to intestinal clearance observed in primary cell Gut/Liver MPS correlates with isoenzyme expression in the human intestine. Image Credit: Zyme Communications Ltd
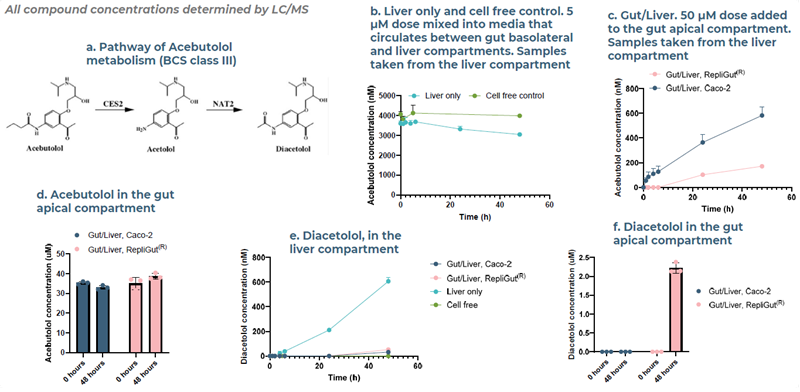
Fig 9. Case study 2, Acebutolol: the combination of intestinal metabolism and low permeability contribute to its low oral bioavailability. This is observed in the primary Gut/Liver MPS and correlates with acebutolol’s bioavailability in humans. Image Credit: Zyme Communications Ltd
Conclusion
- The Gut/Liver MPS is a pre-clinical assay designed to profile human oral bioavailability in vitro.
- Validation criteria ensures donor lots of liver and intestinal cells are functional in co-culture and are metabolically suitable for ADME studies.
- Real-world drug examples demonstrate the assay's utility in providing drug developers with a mechanistic understanding of bioavailability in vitro, allowing the progression of the most promising drug candidates.
References
- Edington, C.D., et al. (2018). Interconnected Microphysiological Systems for Quantitative Biology and Pharmacology Studies. Scientific Reports, [online] 8(1). https://doi.org/10.1038/s41598-018-22749-0.
- Baker, T.K., et al. (2023). The Current Status and Use of Microphysiological Systems by the Pharmaceutical Industry: The IQ Microphysiological Systems Affiliate Survey and Commentary. Drug metabolism and disposition/DMD online, p.DMD-001510. https://doi.org/10.1124/dmd.123.001510.
- Imai, T., et al. (2005). IDENTIFICATION OF ESTERASES EXPRESSED IN CACO-2 CELLS AND EFFECTS OF THEIR HYDROLYZING ACTIVITY IN PREDICTING HUMAN INTESTINAL ABSORPTION. Drug Metabolism and Disposition, 33(8), pp.1185–1190. https://doi.org/10.1124/dmd.105.004226.
About CN Bio
CN Bio is a leading organ-on-a-chip (OOC) company that offers a portfolio of products and contract research services to optimize the accuracy and efficiency of bringing new medicines to market. With more than a decade of research and development experience, they aim to transform the way human-relevant pre-clinical data is generated through the development of advanced in vitro human organ models.
CN-Bio's PhysioMimix® Core microphysiological system (MPS) enables researchers to recreate human biology in the lab and is the only microphysiological system with validated performance across single-, multi-organ, and higher throughput configurations. This easy to adopt, adapt and scale technology bridges the gap between traditional cell culture and human studies, to support the development of safer and more efficacious therapeutics, whilst reducing the dependence on animal model usage.
CN Bio’s portfolio of products (MPS, 3D validated cells, consumable plates) and services support researchers that require reliable, data-rich, in vitro studies, to uncover novel mechanistic insights into drug or disease mechanism of action.
About ELRIG (UK) Ltd.
The European Laboratory Research & Innovation Group (ELRIG) is a leading European not-for-profit organization that exists to provide outstanding scientific content to the life science community. The foundation of the organization is based on the use and application of automation, robotics and instrumentation in life science laboratories, but over time, we have evolved to respond to the needs of biopharma by developing scientific programmes that focus on cutting-edge research areas that have the potential to revolutionize drug discovery.
Comprised of a global community of over 12,000 life science professionals, participating in our events, whether it be at one of our scientific conferences or one of our networking meetings, will enable any of our community to exchange information, within disciplines and across academic and biopharmaceutical organizations, on an open access basis, as all our events are free-of-charge to attend!
Our values
Our values are to always ensure the highest quality of content and that content will be made readily accessible to all, and that we will always be an inclusive organization, serving a diverse scientific network. In addition, ELRIG will always be a volunteer led organization, run by and for the life sciences community, on a not-for-profit basis.
Our purpose
ELRIG is a company whose purpose is to bring the life science and drug discovery communities together to learn, share, connect, innovate and collaborate, on an open access basis. We achieve this through the provision of world class conferences, networking events, webinars and digital content.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.
Last Updated: Dec 12, 2025