The connection between the human liver and the intestine is unique, and the “gut-liver axis” plays a vital role in regulating liver disease pathology.
When homeostasis is disturbed, changes in dietary factors and microbiota can impact intestinal barrier function. This can result in the release of microbial metabolites and components that move to the liver, contributing to inflammation, fibrosis, and cancer.1
It is suggested that the development of Metabolic-dysfunction associated steatotic liver disease (MASLD), previously known as non-alcoholic fatty liver disease (NAFLD), is closely associated with alterations in the gut-liver axis.2
There are currently no FDA-approved medications for treating MASLD and only one for its more severe form, Metabolic-dysfunction associated steatohepatitis (MASH), previously known as Nonalcoholic steatohepatitis (NASH). Understanding the mechanisms that drive disease progression is essential for informing therapeutic R&D programs.
The presently available preclinical models of MASLD and MASH, whether in vivo or in vitro, have several limitations and do not fully represent the important aspects of the human disease state.3
Few models accurately depict the human gut-liver axis and allow for studies into the molecular mechanisms driving MASLD progression.
A fully human microphysiological system (MPS) model was developed for the liver and intestine, as well as a multi-organ MPS platform that connects the intestine and liver. These advanced in vitro models enable the investigation of MASLD/MASH and the gut-liver axis.
Aims
- To show that a liver MPS can be prompted to replicate essential features of a MASLD/MASH phenotype. Additionally, demonstrate that the model responds to the FXR agonist - Obeticholic acid (OCA), which is a semi-synthetic bile acid analogue.
- Illustrate that the intestine-liver MPS can crosstalk and imitate the oral absorption of therapeutic compounds.
- Demonstrate that treating the intestine-liver MPS with OCA leads to physiological responses in both tissues.
Materials and methods
Cryopreserved primary human hepatocytes (PHH), human hepatic stellate cells (HSC), and human Kupffer cells (HK) were acquired from Life Technologies (USA) and cultured in HEP-Fat or HEP-Lean medium.
The cells were co-cultured at physiologically pertinent ratios in the PhysioMimix® System for up to 15 days. Fat accumulation was assessed by Oil Red O staining of fixed microtissues and then normalized to the total protein content.
The production of IL-6, TNFα, and albumin was measured through ELISA (R&D systems). Cytokine profiles in cell culture samples were analyzed using Luminex analysis with the Milliplex map Human cytokine/chemokine magnetic bead panel – premixed 38 plex (Merck Millipore).
Fibrosis in 3D microtissues was stained and imaged using a Yokogawa CV7000 high-content imaging system. The data produced was computed using automated MatLab scripts. For transcriptomic analysis, RNA-seq or QPCR was employed to analyze cDNA, utilizing an Illumina NextSeq500 benchtop sequencer or QuantStudio 6 analyzer.
Microtissues for intestine MPS were formed using CacoGoblet (ReadyCell) Transwells and cultured with primary hepatocytes in PhysioMimix® plates in optimized MPS media.
CYP activity and drug metabolism data were produced through quantitative LC-MS analysis. TEER values were measured using an EVOM2 probe (World Precision Instruments).
Results
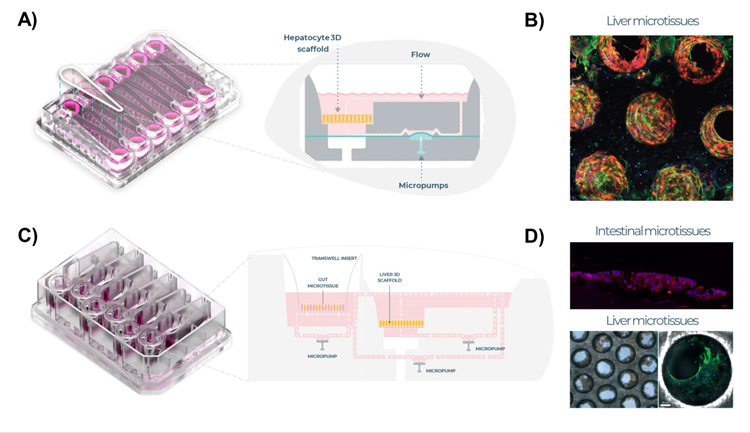
Figure 1. Liver MPS and intestine liver MPS set up. A) Liver MPS designed for PhysioMimix® system, uses open well plates (PhysioMimix® Multi-chip Liver-12 plates) for the culture of primary liver cells in a 3D engineered scaffold, with 12 replicates per plate. Schematic representation of each replicate shown. B) Highly functional 3D liver microtissue formed over time within embedded scaffolds (IF staining: red - albumin, green - cytoskeleton). C) Intestine-liver MPS (PhysioMimix®) with six replicates per plate and fluidic link between the liver and intestine MPS, schematic representation shown of replicate. D) Intestinal microtissues cultured on Transwell® inserts (IF staining: red - ZO-1 and liver microtissues (equivalent to those cultured in the liver MPS) were cultured in the Dual-organ MPS. Image Credit: CN Bio
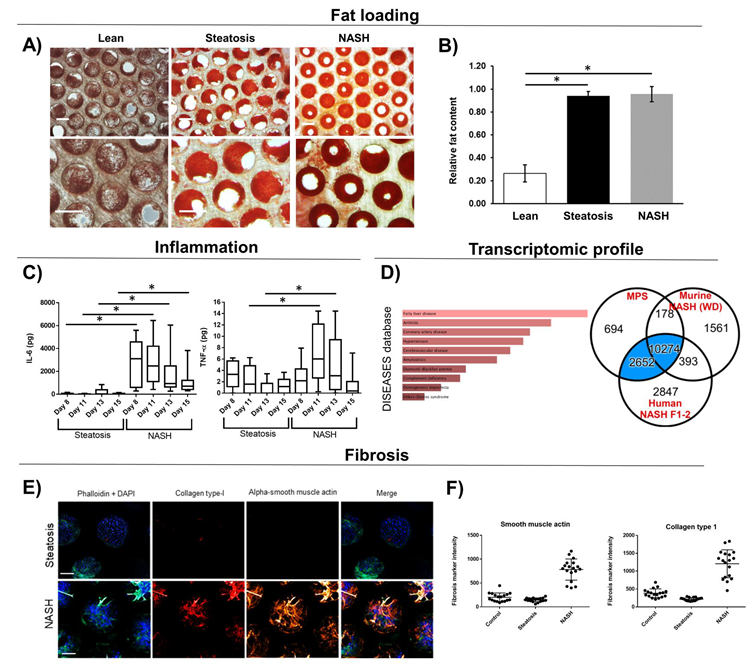
Figure 2. Liver MPS microtissues loaded with fat display MASH phenotype. PHH, HK and HSC were cultured in the liver MPS in either lean or fat conditions. Steatosis controls contained 10-fold less HK and HSC than MASH co-cultures. Microtissues analyzed for A + B) Fat loading via quantified Oil Red O staining. C) Inflammatory biomarkers were measured in cell culture medium; D) Transcriptomic profile of NASH microtissues was analyzed via DISEASEs database and compared to the profile of human MASH biopsy samples and western diet (MASH mice - numbers highlight number of overlapping DEGs. E) Fibrosis was measured and quantified using high content confocal microscopy. Microtissues were imaged (representative images shown) and F) images quantified. All scale bars 300 µm. Data are mean ± SD, n = minimum 6. * = P < 0.05. Image Credit: CN Bio Innovations Limited
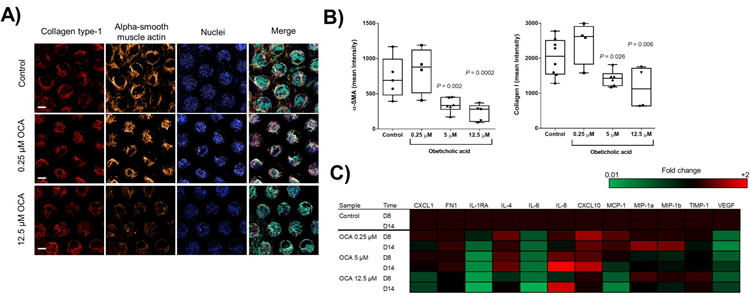
Figure 3. OCA treatment of liver MPS microtissues reduces MASH phenotype. Liver microtissues were cultured in the Liver MPS for 15 days and after 4 days cultures were dosed daily with the FXR agonist OCA or vehicle 0 1% DMSO). A) Confocal microscopy images of microtissues from each condition at the end of the study (representative images shown). For each scaffold, 8 regions of interest were acquired and each biological condition has N= 4. B) Quantification of staining for collagen type 1 and alpha SMA in microtissues. Data are mean ± SD, N = 4. * = P < 0.05. C) Key cytokines expressed in different conditions at two time points during the experiment, shown as Log2 fold change compared to vehicle control samples. Image Credit: CN Bio
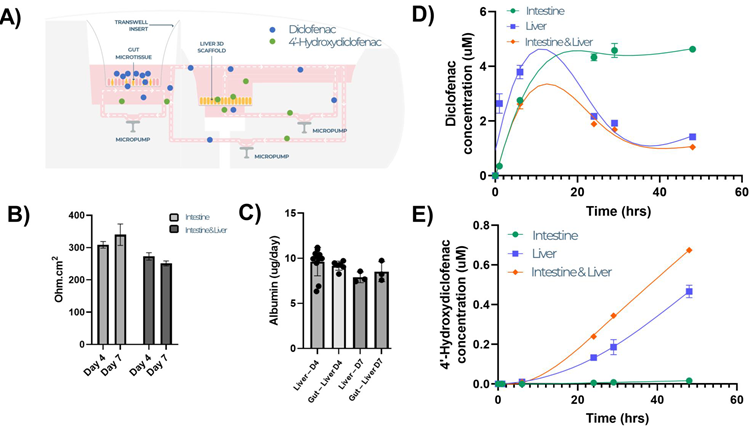
Figure 4. Intestine liver multi-organ MPS mimics the human gut liver axis and enables inter-organ crosstalk and first-pass metabolism studies. Intestinal and liver microtissues were co-cultured in the Dual-Organ plate for seven days and dosed with Diclofenac (10 µM) in the apical compartment of the intestine MPS (to mimic an oral dose) and cultured for 48 hours. A) Schematic representation of transfer of Diclofenac and its primary metabolite between the two tissue models. B) TEER measurements of intestinal culture, C) albumin production by liver culture. Concentration of Diclofenac (D) and its primary metabolite 4-hydroydiclofenac (E) were measured by LC-MS in the circulating medium across time, demonstrating drug absorption and metabolism in the same linked MPS platform The MPS was run with single- organ and multi-organ set-ups for comparison All data shown is a minimum of three biological replicates and shown as mean ± SD. Image Credit: CN Bio
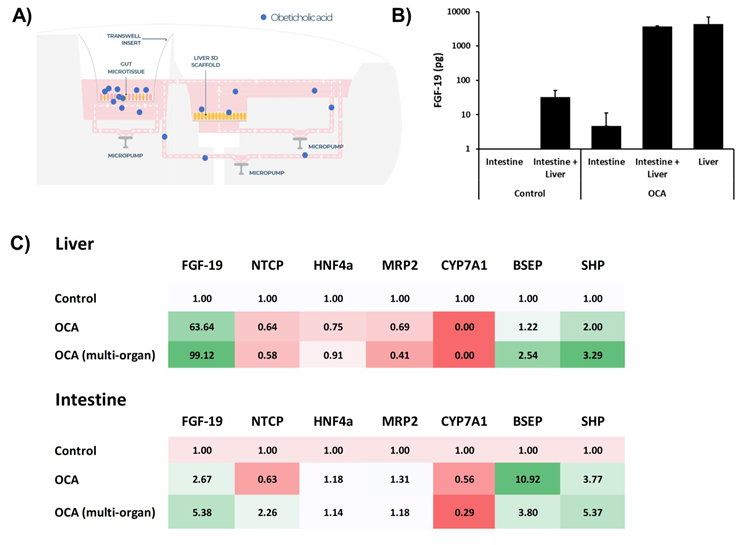
Figure 5. Treatment of intestine-liver MPS with FXR agonist induces synergistic responses from both organ models. Liver MPS and intestine liver MPS were cultured for seven days, in either the PhysioMimix Liver-12 or Dual-organ plates and dosed with FXR agonist OCA - 20 µM in the apical compartment of the intestine MPS (to mimic an oral dose) and cultured for 48 hours. A) Schematic representation of the transfer of OCA between the two tissue models. B) Fibroblast growth factor (FGF 19) production measured via ELISA from single or multi-organ cultures with and without treatment. C) Transcriptomic changes to key FGF-19 target genes in liver and intestinal tissue following OCA exposure. Data shown as fold change compared to control/untreated tissue. All data is a minimum of three biological replicates and shown as mean ± SD. Image Credit: CN Bio
Conclusions
By using the PhysioMimix® system, a fully human in vitro model of MASH was established in this study.
Co-cultures of PHH, HK, and HSC were cultured in fat-containing medium, inducing important features of clinical disease such as inflammation, fat loading, and fibrosis. The transcriptional profile of this model closely resembled that of human F1-2 MASH patients, more so than the profile of Western diet mice.
When the MASH Liver MPS were treated with OCA, clear dose-responsive effects on both inflammatory and fibrotic endpoints were successfully demonstrated.
The dual-organ intestine-liver MPS’s capability to maintain the linked tissue models for over one week of culture was also demonstrated, recapitulating first-pass metabolism after oral drug delivery.
The dosing of the multi-organ MPS with OCA induced synergistic responses, with more robust effects observed in intestinal and liver tissues when co-cultured together rather than in isolation.
OCA dosing on the intestinal cultures resulted in substantial FGF-19 production and a notable change in gene expression, especially in the liver microtissues. This included a loss of CYP7A1 expression and an upregulation of BSEP and SHP, mirroring in vivo observations.
This study shows that these MPS model(s) are ideal for exploring the molecular mechanisms underlying MASH development and can serve as valuable tools for evaluating the effectiveness of innovative therapeutic strategies.
References
- Ohtani, N., & Kawada, N. (2019). Role of the Gut–Liver Axis in Liver Inflammation, Fibrosis, and Cancer: A Special Focus on the Gut Microbiota Relationship. Hepatology Communications (Vol. 3, Issue 4, pp. 456–470).
- Marra, F., & Svegliati-Baroni, G. (2018). Lipotoxicity and the gut-liver axis in NASH pathogenesis. Journal of Hepatology (Vol. 68, Issue 2, pp. 280–295).
- Santhekadur, P. K., Kumar, D. P., & Sanyal, A. J. (2018). Preclinical models of non-alcoholic fatty liver disease. Journal of Hepatology, 68(2), 230–237.
About CN Bio
CN Bio is a leading organ-on-a-chip (OOC) company that offers a portfolio of products and contract research services to optimize the accuracy and efficiency of bringing new medicines to market. With more than a decade of research and development experience, they aim to transform the way human-relevant pre-clinical data is generated through the development of advanced in vitro human organ models.
CN-Bio's PhysioMimix® Core microphysiological system (MPS) enables researchers to recreate human biology in the lab and is the only microphysiological system with validated performance across single-, multi-organ, and higher throughput configurations. This easy to adopt, adapt and scale technology bridges the gap between traditional cell culture and human studies, to support the development of safer and more efficacious therapeutics, whilst reducing the dependence on animal model usage.
CN Bio’s portfolio of products (MPS, 3D validated cells, consumable plates) and services support researchers that require reliable, data-rich, in vitro studies, to uncover novel mechanistic insights into drug or disease mechanism of action.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.