Retinoic acid is a member of the over 4,000-strong family of retinoids, which are compounds derived from retinol or vitamin A, or compounds structurally similar to it.
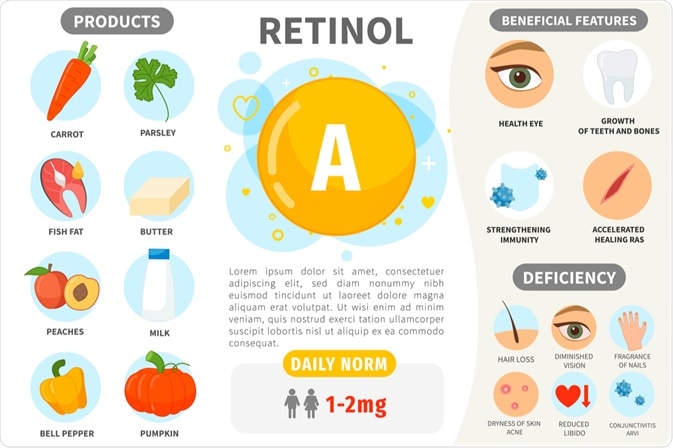
Image Credit: Igdeeva Alena / Shutterstock.com
How is retinoic acid formed?
Vitamin A itself is derived only from food, and cannot be made in the body of any animal. It is esterified and stored in the liver, which is the richest source of vitamin A apart from supplements.
Retinoic acid is synthesized from retinol via two enzymatic reactions, the first of which is a reversible oxidation of retinol to retinal, which is followed by a second and irreversible oxidation reaction to retinoic acid.
Retinoic acid is a light-sensitive compound, like other retinoids, because of the alternating double bonds between the carbon atoms in its hydrophobic tail, which is attached to a 6-carbon ring. The low molecular weight of the compound also makes it highly fat-soluble, which means it easily diffuses across cell membranes.
Functions
Retinoids are important biological molecules that participate in cell growth, epithelial cell growth and maturation, apoptosis, and immunologic function. Furthermore, retinoic acid is vital in embryonic life for organ development, as well as visual function. Retinoids like retinoic acid help to transform cell types from the proliferative profile to the maturation profile by inducing differentiation.
Retinoic acid is bound in the cell by cellular retinoic acid-binding protein (CRABP) and inside the nucleus by two types of receptors, the retinoic acid receptor (RAR) and the retinoid X receptor (RXR). There are various biologically active forms of retinoic acid that isomerize under physiological conditions. The different isomers act on different receptors.
Retinoic acid and health conditions
All retinoids have a variety of functions in visual and skin conditions, and in cancer therapeutics. A low vitamin A intake is associated with higher cancer risk. Abnormal RA receptors are also linked to cancer development.
Both animal and human studies have demonstrated that retinoids suppress cancerous changes. More specifically, human studies have confirmed that retinoid administration suppresses cancers of the breast, lung, and liver. These studies have also found that retinoids can reverse premalignant changes and induce differentiation of the myeloid cell series in blood.
All-trans RA, or tretinoin, is the most abundant natural isoform and has been found to be an active agent against a wide range of cancers of the lung, brain, kidney, blood cells, lymphatic tissue, uterine cervix, and skin. Another form of retinoic acid known as 9-cis retinoin, or isotretinoin, is also used in the treatment of skin lesions of Kaposi’s sarcoma, as well as for cancers of the breast and prostate.
Vitamin A is found in all dark green, yellow, and red vegetables as well as red or yellow non-citrus fruits. This important vitamin is also richly found in fish like sardines and cod, as well as in liver and fish oils.
Retinoic acid has been extensively used to prevent and treat photoaged skin and psoriasis. Both etretinoin and isotretinoin, which are two synthetic forms of vitamin A, are highly teratogenic. Etretinoin is not available for medical use, while isotretinoin is strictly controlled.
References
Further Reading
Last Updated: Mar 30, 2021