A receptor-binding domain (RBD) is a key part of a virus located on its ‘spike’ domain that allows it to dock to body receptors to gain entry into cells and lead to infection. These are also the primary targets in the prevention and treatment of viral infections, including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) – the virus that causes COVID-19.
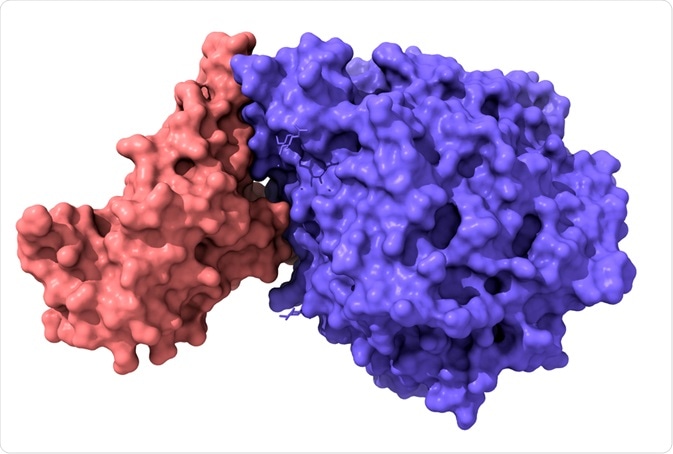 Model of structure of novel coronavirus spike receptor-binding domain (pink) complexed with its receptor ACE2 (blue). Image Credit: Volodymyr Dvornyk/Shutterstock.com
Model of structure of novel coronavirus spike receptor-binding domain (pink) complexed with its receptor ACE2 (blue). Image Credit: Volodymyr Dvornyk/Shutterstock.com
What is a receptor-binding domain (RBD)?
A receptor-binding domain (RBD) is a short immunogenic fragment from a virus that binds to a specific endogenous receptor sequence to gain entry into host cells. Specifically, these refer to a part of the ‘spike’ glycoprotein (S-domain) which is needed to interact with endogenous receptors to facilitate membrane fusion and delivery to the cytoplasm. Typically, the S-domain is also the site of neutralizing antibodies.
SARS and MERS
The entry of beta-coronaviruses, such as severe acute respiratory syndrome coronavirus (SARS-CoV), the virus that causes SARS, requires the binding of its spike glycoprotein, ‘S’ domain, to the ACE2 receptor in the body. Coronaviruses are dotted with such S domains all over their surface giving the appearance of a large distinctive ‘crown’ appearance and thus the name ‘corona’/crown, viruses.
Each S domain is around 20 nm surface projection that surrounds the periphery of the coronavirus and varies considerably between different coronaviruses. Within the S domain of SARS-CoV, there is a short domain (within the S1 subunit) containing just 2 glycosylation sites that secrete short fragments of RBD (by glycosylation) that fold and bind to the ACE2 receptor.
One functional glycosylation site is sufficient to create an RBD fragment that can bind to ACE2. Specific mutations within RBD amino acid residues (e.g. K390) lead to decreased affinity to ACE2. Thus, the binding of the viral RBD on its S-domain is an essential step for membrane fusion and entry into target host cells, as it leads to the S2 domain to transit from a pre-fusion state to a stable post-fusion state anchoring it to the membrane.
Concerning Middle East respiratory syndrome-related coronavirus (MERS-CoV), the virus that causes MERS, the key difference between MERS-Cov and SARS-CoV is the viral S-domain (S1). In MERS-CoV, a 1353-amino acid type I membrane glycoprotein assembles into trimers forming the ‘spikes’ which bind and fuse to the DPP4/CD26 receptor.
The MERS-CoV RBD (E367 and Y606) forms a complex with DDP-4’s extracellular domain (S39-P766). Thus, whilst the core CoV domains remain very similar, their RBD sequence varies considerably, influencing differing receptor affinity.
.jpg) SARS-CoV-2 illustration. Image Credit: Kateryna Kon/Shutterstock.com
SARS-CoV-2 illustration. Image Credit: Kateryna Kon/Shutterstock.com
SARS-CoV-2 (COVID-19)
Similar to SARS-CoV, SARS-CoV-2 (the virus that causes COVID-19) binds to the ACE2 receptor to gain entry into respiratory and digestive epithelial cells. The S-domain of SARS-CoV-2 contains an RBD which enables it to bind to ACE2 and fuse into the membrane of epithelial cells.
The RBD of SARS-CoV-2’s S1 domain strongly binds to both human and bat ACE2. SARS-CoV-2’s RBD is located between residues 331-524 (or Thr333-Gly526 in a different study) of the S1 domain, which enables it to bind to ACE2 – and more strongly than SARS-CoV (according to some studies) – which may reflect the higher infectiousness of SARS-CoV-2 compared to SARS-CoV.
The SARS-CoV-2 RBD has a twisted 5-stranded antiparallel beta-sheet with short connecting helices and loops. In the core, between the β4-7 strands, there is an additional extended insertion containing short β5-6 strands. This extension is where the receptor-binding motif (RBM) is, which contains the contacting residues that enable it to bind to ACE2.
The identification of the RBD can help lead to the development of monoclonal (or polyclonal) antibodies (vaccines) in the treatment and prevention of coronaviral infection, as the RBD is the site for many major neutralizing antibodies – preventing the virus binding to the receptor (ACE2/DPP4).
Studies have shown that SARS-CoV RBD-specific polyclonal antibodies can cross-react with SARS-CoV-2 RBD protein to inhibit its entry into ACE2 in vitro (human expressing cells). Furthermore, SARS-CoV RBD-polyclonal antibodies can also cross-neutralize SARS-CoV-2 pseudoviral infection leading to the possibility of creating a SARS-CoV-RBD-based vaccine for SARS-CoV-2 prevention.
Thus, convalescent plasma from recovered SARS and COVID-19 patients is being looked at in terms of treatment and prevention for COVID-19. Many studies are being carried out on convalescent plasma in COVID-19, and are suggesting that it is not effective later on in severe, hospitalized disease but may still be an option for early on or preventative treatment.
Many antibodies raised against SARS-CoV have shown mixed results concerning SARS-CoV-2, with only one (CR3002) which can bind to both that targets a portion of SARS-CoV-2 RBD.
Convalescent plasma from recovered SARS patients which does cross-react with SARS-CoV-2 requires a more in-depth analysis of the sequence of the RBD that is affected in the development of strong and successful preventative vaccinations. Thus, studying the RBD of SARS-CoV-2 is important for successful therapies and vaccinations. Many different vaccinations have now been successfully created and administered for SARS-CoV-2.
Neutralizing Antibody? Discover tests targeting the receptor binding domain of the spike protein
Summary
In summary, an RBD is a critical component of the viral spike glycoprotein that is found on coronaviruses including SARS-CoV-2, the virus that causes COVID-19. The binding of the RBD on the spike domain is a critical step that allows coronaviruses to bind to target body receptors (such as ACE2 on respiratory epithelial cells) and enter cells to cause infection.
The RBD is therefore an important target for neutralizing antibodies – either through engineered vaccination or convalescent plasma of recovered patients. Whilst many vaccinations are now being administered across the globe (March 2021), convalescent plasma trials are having mixed results.
References
- Chakraborti et al, 2005. The SARS Coronavirus S Glycoprotein Receptor Binding Domain: Fine Mapping and Functional Characterization. Virology Journal 2(73). https://virologyj.biomedcentral.com/articles/10.1186/1743-422X-2-73
- He et al, 2004. Receptor-binding domain of SARS-CoV spike protein induces highly potent neutralizing antibodies: implication for developing subunit vaccine. Biochem Biophys Res Commun. 324(2): 773–781. https://pubmed.ncbi.nlm.nih.gov/15474494/
- Mou et al, 2013. The Receptor Binding Domain of the New Middle East Respiratory Syndrome Coronavirus Maps to a 231-Residue Region in the Spike Protein That Efficiently Elicits Neutralizing Antibodies. J Virol. 87(16): 9379–9383. https://pubmed.ncbi.nlm.nih.gov/23785207/
- Tai et al, 2020. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for the development of RBD protein as a viral attachment inhibitor and vaccine. Cell Mol Immunol. 17(6): 613–62. https://pubmed.ncbi.nlm.nih.gov/32203189/
- Lan et al, 2020. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 581(7807):215-220. https://pubmed.ncbi.nlm.nih.gov/32225176/
- Premkumar et al, 2020. The receptor-binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients. Sci Immunol. 5(48):eabc8413 https://pubmed.ncbi.nlm.nih.gov/32527802/
Further Reading
Last Updated: Mar 8, 2021