Enzyme mechanisms of catalysis play many important roles in the functioning of a healthy body. An enzyme may exist as a multi-subunit complex or it may be associated with a cofactor such as adenosine triphosphate (ATP). Enzymes are vital for biochemical reactions that occur at a very slow rate at room temperature or pressure because they can drive the reaction even under otherwise normal conditions.
Principles of enzyme catalysis
In order for a reaction to take place, the reactant molecules need to contain sufficient energy to reach the activation energy (Ea) of the reaction, which is equal to the energy required for the intermediates to reach the transition state.
Each molecule possesses a unique amount of energy depending on the recent reactions they have undergone and environmental factors, such as the temperature and pressure in the area.
For reactions with a high Ea, it is unlikely that they will occur to a great extent under normal conditions. In this case, enzymes can assist the reaction by lowering the activation energy that is required for it to occur so that the reaction can be fulfilled more easily.
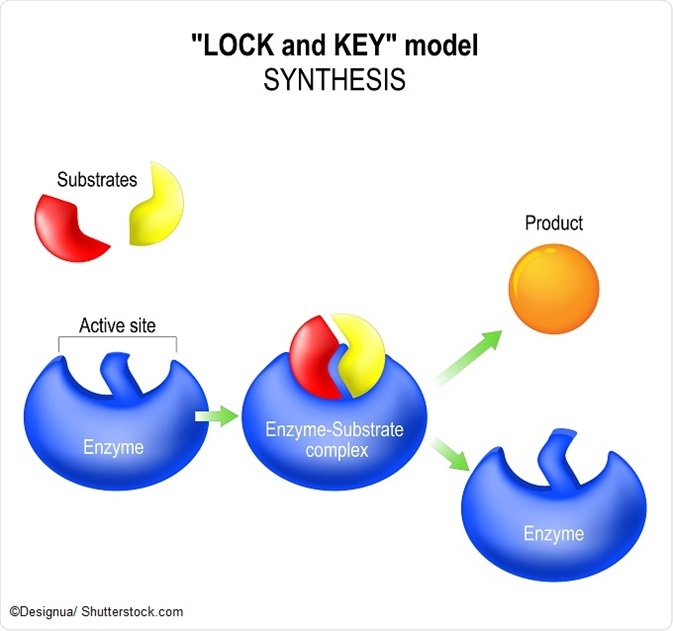
A simplified equation of the enzyme equation is as follows:
E + S ⇔ ES ⇔ EP⇔ E+P
This shows that the enzyme and substrate are both present at the beginning of the reaction, and then form a complex. This allows the product to be formed due to the reduction in activation energy of the enzyme and the product can finally dissociate in the final step. It is worth noting that the enzyme is present both at the beginning and the end of the equation – it is not depleted – and can hence be reused for subsequent enzyme equations.
Enzyme mechanisms to reduce activation energy
There are several different mechanisms that can lead to a reduction in activation energy so that the reaction can occur.
In many cases, the enzyme binds to the substrate so that the active complex can react. This involves a reduction in the activation entropy due to the loss of translational and rotational entropy. Other mechanisms may work by:
- Straining the reactants so that there is more binding energy available for the transition state.
- Provision of an alternative reactive pathway
- Desolvation of ionic groups responsible for the catalysis and reaction
The mechanisms of enzyme catalysis are very similar to other types of catalysis. They simply provide an alternative route for the reaction to occur so that less energy is required for the intermediates to reach the transition state. This means that it reduces the activation energy (Ea) of the reaction so that it can be more easily reached. The catalyst is not consumed in the mechanism but may be reused in many catalysis reactions.
Enzyme interactions
There are various ways that the enzyme and substrates may interact to assist the reaction, which are covered in this section.
The induced fit model involves an initially weak interaction between the enzyme and the substrate, which is quickly strengthened by conformational changes in the enzyme. This is the favored mechanism of enzyme catalysis because it produces a strong and stable form of enzyme binding. There are two primary ways that the substrate may bind to the enzyme: uniform binding and differential binding.
An enzyme may help align the molecules to increase proximity and encourage the correct orientation for the reaction, which reduced the entropy of the reactants. Additionally, an enzyme may act as a proton donor or acceptor to stabilize the molecules in the transition state, thus lowering the activation energy of the reaction. Stabilization of charged transition state via electrostatic catalysis is another mechanism of enzymes to function. The enzyme residue may form a transient covalent bond with the substrate, which is a mechanism known as covalent catalysis. Finally, other mechanisms of enzyme catalysis include metal ion catalysis, bond strain, and active enzyme.
References
Further Reading
Last Updated: Jul 19, 2023