Protein folding is a process by which a polypeptide chain folds to become a biologically active protein in its native 3D structure. Protein structure is crucial to its function. Folded proteins are held together by various molecular interactions.
During translation, each protein is synthesized as a linear chain of amino acids or a random coil which does not have a stable 3D structure. The amino acids in the chain eventually interact with each other to form a well-defined, folded protein. The amino acid sequence of a protein determines its 3D structure. Folding of proteins into their correct native structure is key to their function. Failure to fold properly produces inactive or toxic proteins that malfunction and cause a number of diseases.
Four stages of protein folding
The folding of a protein is a complex process, involving four stages, that gives rise to various 3D protein structures essential for diverse functions in the human body. The structure of a protein is hierarchically arranged, from a primary to quaternary structure. The wide variation in amino acid sequences accounts for the different conformations in protein structure.
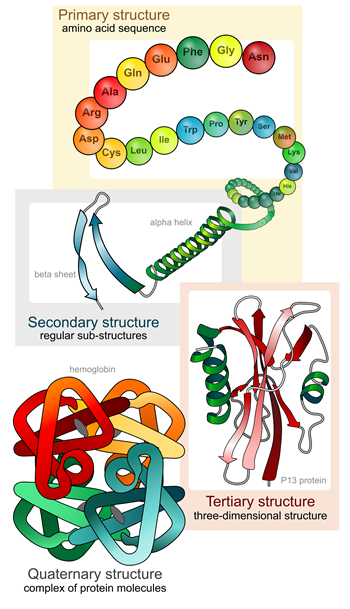 Primary structure refers to the linear sequence of amino-acid residues in the polypeptide chain.
Primary structure refers to the linear sequence of amino-acid residues in the polypeptide chain.- Secondary structure is generated by formation of hydrogen bonds between atoms in the polypeptide backbone, which folds the chains into either alpha helices or beta-sheets.
- Tertiary structure is formed by the folding of the secondary structure sheets or helices into one another. The tertiary structure of protein is the geometric shape of the protein. It usually has a polypeptide chain as a backbone, with one or more secondary structures. The tertiary structure is determined by the interactions and bonding of the amino acid side chains in the protein.
- Quaternary structure results from folded amino-acid chains in tertiary structures interacting further with each other to give rise to a functional protein such as hemoglobin or DNA polymerase.
Factors affecting protein folding
Protein folding is a very sensitive process that is influenced by several external factors including electric and magnetic fields, temperature, pH, chemicals, space limitation and molecular crowding. These factors influence the ability of proteins to fold into their correct functional forms.
Extreme temperatures affect the stability of proteins and cause them to unfold or denature. Similarly, extreme pH, mechanical forces and chemical denaturants can denature proteins. During denaturation, proteins lose their tertiary and secondary structures and become a random coil. Although denaturation is not always reversible, some proteins can re-fold under certain conditions.
Some cells contain heat shock proteins or chaperones that protect proteins in the cell against heat denaturation. Chaperones help proteins to fold and remain folded under extreme temperatures. They also assist misfolded proteins in unfolding and re-folding correctly.
Diseases related to incorrect protein folding
Misfolded proteins denature easily and lose their structure and function. Incorrect protein folding can lead to many human diseases.
Alzheimer's disease is an example of a neurodegenerative condition caused by protein misfolding. This disease is characterized by dense plaques in the brain caused by misfolding of the secondary β-sheets of the fibrillar β-amyloid proteins present in brain matter. Huntington's disease and Parkinson's disease are other examples of neurodegenerative diseases associated with protein misfolding.
Cystic fibrosis (CF) is a fatal disease caused by misfolding of the cystic fibrosis transmembrane conductance regulator (CFTR) protein. In most cases of CF, the phenylalanine at position 508 of the CFTR is deleted, causing misfolding of the regulator protein. Several allergies have also been shown to be caused by incorrect protein folding.
References
- http://www.ncbi.nlm.nih.gov/pubmed/10494843
- http://lectures.molgen.mpg.de/ProteinStructure/Levels/
- http://www.chemicalconnection.org.uk/chemistry/topics/view.php?topic=5&headingno=6
- http://chemwiki.ucdavis.edu/Core/Biological_Chemistry/Proteins/Protein_Structure/Protein_Folding
Further Reading
Last Updated: Feb 26, 2019