What is an Antibody?
General Structure of an Antibody
Classification of Antibodies
Exceptions to the Rule
References
What is an Antibody?
Vertebrates with a defined immune system, such as humans, have an immune response consisting of an innate and adaptive response, also known as a specific response. The specific response is tailored to the pathogen infecting the body and targets it specifically. The innate, or nonspecific, response combats any pathogen it encounters.
Antibodies, also known as Immunoglobulins, are incredibly specific molecules that bind to their target antigen and neutralize it in the most common cases. They achieve this by promoting a change in structure, blocking binding sites needed to complete a function, or marking out the cell they are bound to for phagocytosis. They can also aid the precipitation of antigens or the gathering of cells.
When detected, the body generally produces them in response to toxins or other pathogenic items. They are part of the specific immune response and are generated to combat known threats. Antibodies can also play a therapeutic role and are used to influence specific metabolic pathways.
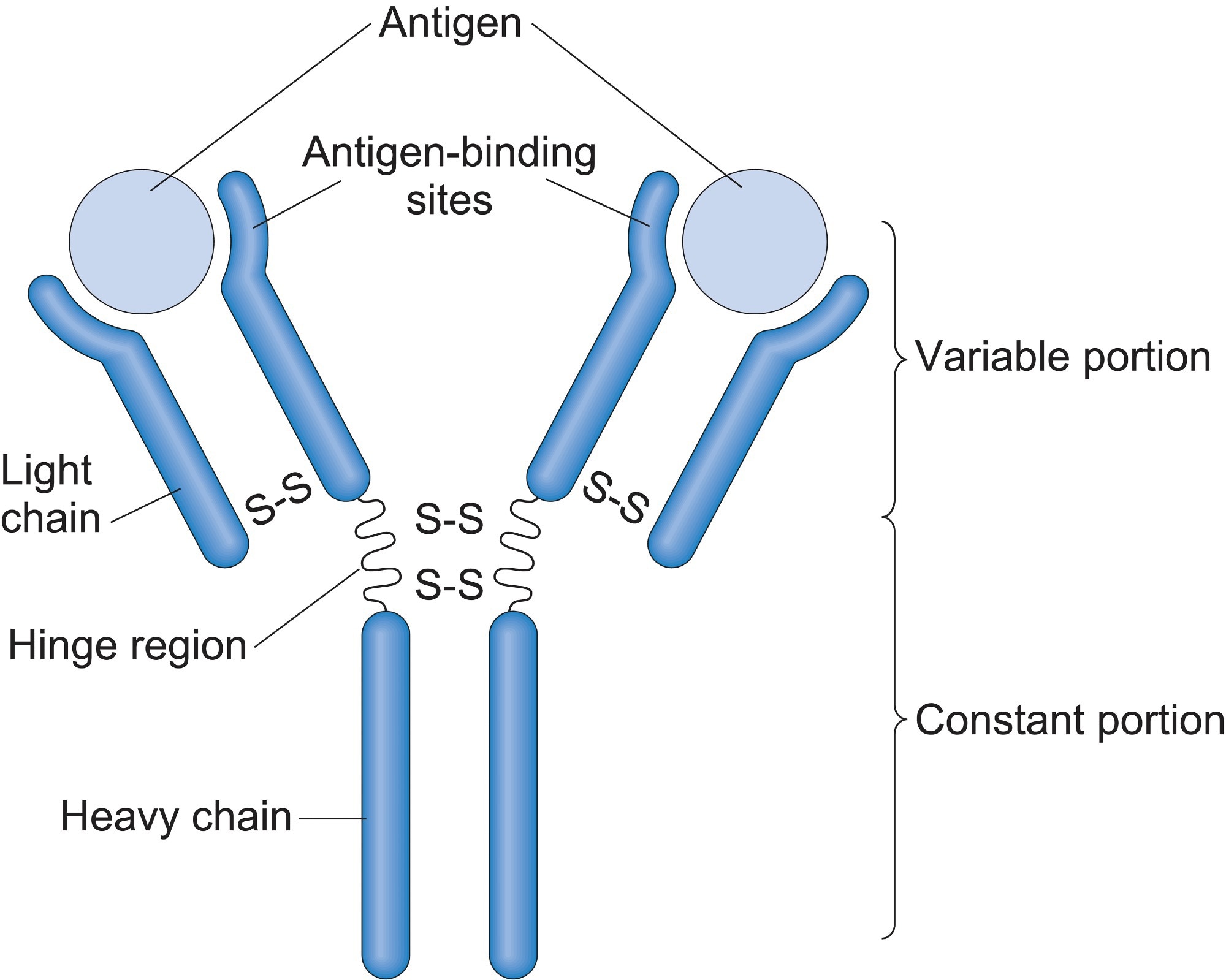
Image Credit: periyanayagam/Shutterstock.com
General Structure of an Antibody
Immunoglobulin (Ig) molecules are "Y" shaped glycoproteins that consist of two heavy and two light chains. The light chains are made up of two domains, and the heavy ones are made up of four. These domains all have constant and variable regions accounting for the wide variations in structure seen between molecules.
The arms of the "Y" form are made up of light chains, and the N-terminal of the heavy chains tightly bound together. The body of the "Y" is formed by the other ends of the heavy chains, the C-terminals.
They are divided into the classes A, D, E, G, and M, the most common of which is IgG. This form is commonly found in blood and tissues, easily crossing the placenta and offering the first immunity to the fetus. Class IgG has four subclasses that vary slightly in structure, but each has two identical antigen binding sites at the ends of their "Y" arms.
The heavy and light chains are folded into the same beta-sheet general structure but show much variation in the smaller details. The most variable sections of the antibody are the antigen binding sites which are a mix of the variable regions of both light and heavy chains. These binding sites define the different types of antibodies and their function.
The sequences primarily responsible for the antigen binding sites specificity are three highly variable gene recombining regions (CDR) loops located at the antigen binding site at the end of the "Y" arms. The arm of the "Y" structure is linked to the stem via a uniquely structured peptide hinge made up of parallel poly-proline helices.
The light chains that make up the antibody have a variable and a constant region, whereas the heavy chains consist of three variable and one constant region. This aids the stem in binding to the immune receptors located on the cell surface and going on to elicit an immune response specific to the type of antibody bound.
Another factor that adds to the specificity of each antibody is the addition of glycan molecules. These molecules ensure that the antibody binds to the correct Fc receptor on the surface of cells. The Fc receptors are present on the surface of immune cells such as macrophages and neutrophils. The binding initiates the destruction of the invading pathogenic cell.
Classification of Antibodies
The specificity of antibodies means they can be divided into five distinct groups, also known as isotopes or classes, depending on functional and structural differences. Some of these classes contain subtypes based on other differences.
The different isotopes are grouped according to the antibody's type of heavy chain. This affects the number of Ig-like domains, the oligomeric state, and the number of disulfide bonds that form. IgA contains α chains, IgD δ chains, IgE is made up of ε chains, IgG has γ heavy chains, and IgM has μ chains making up their stems.
In the cases of IgA and IgG, they are further divided into subgroups when found in humans. IgA is divided into two further groups, numbered accordingly, and IgG is divided into a further four subclasses.
The distinct classes of antibodies contain light chains from either the κ or λ class, with the majority of antibodies found in the body's systems and used in a clinical capacity consisting of κ light chains.
Image Credit: ustas7777777/Shutterstock.com
Exceptions to the Rule
Although most antibodies have the classic structure consisting of two heavy and two light chains, there are still exceptions found in other species. Antibodies containing only heavy chains have been found in fish with cartilage skeletons and camelids such as camels and llamas.
Another exception to the common structure of antibodies is found in birds. The antibody IgY contains heavy chains with four constant domains instead of the accepted three variable regions and one constant.
References:
- Goulet, D. R., & Atkins, W. M. (2020). Considerations for the Design of Antibody-Based Therapeutics. Journal of pharmaceutical sciences, 109(1), 74–103. https://doi.org/10.1016/j.xphs.2019.05.031
- Huber, R. (1980) Spatial structure of immunoglobulin molecules. Klinische Wochenschrift, 58(22), 1217–1231. https://doi.org/10.1007/BF01478928
- Ma, H., & O'Kennedy, R. (2015). The Structure of Natural and Recombinant Antibodies. Methods in molecular biology (Clifton, N.J.), 1348, 7–11. https://doi.org/10.1007/978-1-4939-2999-3_2
Further Reading
Last Updated: Jul 15, 2022