CDK5 is a member of the cyclin-dependent kinase family that differs from the other CDK proteins in its function and targets. In Alzheimer’s disease, CDK5 phosphorylates Tau, leading to the accumulation of tau within the axons of nerve cells and eventually, apoptosis.
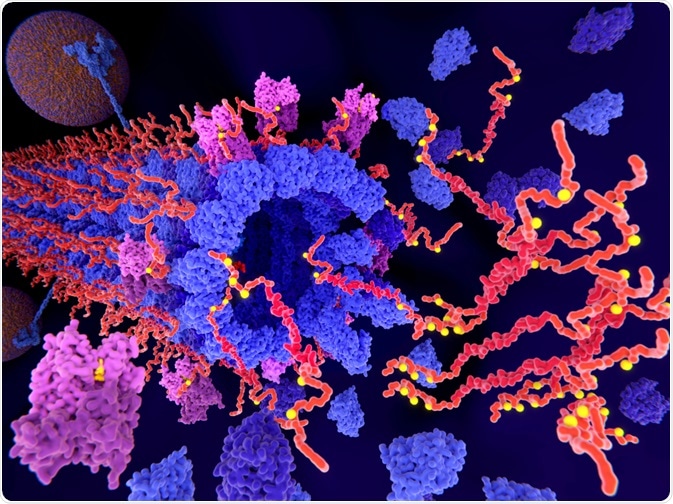
The pathological phosphorylation (yellow) of Tau proteins (red) by CDK5 (violet) leads to the disintegration of microtubuli in an axon and aggregation of tau. (Juan Gaertner | Shutterstock)
What are CDK5 and Tau?
CDK5
CDKs are serine/threonine protein kinase proteins typically involved in cell cycle progression in eukaryotic cells. However, CDK5, also called NCLK (neuronal C2CD-like kinase), is involved in neuronal activity, neuronal developmental migration, and growth of neurites.
CDK5 is expressed at basal levels in most tissues in mammals, but can also be found in the brain and developing muscle tissue. CDK5 deficiency can lead to the defective formation of the cerebellum, and its activator p35 is critical for normal brain development. CDK5 has a diverse range of substrates, including proteins implicated in cytoskeleton dynamics, cell adhesion, and membrane trafficking.
Tau
Tau proteins are primarily expressed in mammalian neurons, where they bind microtubules in the axons. They bind to microtubules and promote its assembly, with additional functions in signaling and cytoskeletal organization being recently discovered. Tau activity is regulated by its phosphorylation. Hyperphosphorylation of its aggregates are implicated in several neurodegenerative diseases, known as taupathy.
How does CDK5 affect Tau?
CDK5 phosphorylation of Tau leads to reduced binding to the microtubule. Thus, it cannot promote assembly of the microtubule, leading to decrease in microtubule nucleation. Such alterations in the stability of the microtubule network, driven by CDK5 phosphorylation by other MAPs, affects neuronal migration during development.
Tau in the nervous system is activated by binding neuronal activators, p35 and p39. P35 additionally releases p25 which can bind to CDK5 via calpain decomposition. The CDK5/p25 complexes promote Tau phosphorylation due to their strong enzymatic effect and hyperactivation of CDK5 by p25. CDK5 is known to phosphorylate Tau at around 9 to 13 sites, including serine residue 396 and threonine residue 231 in disease states.
CDK5/Tau in diseases
Neurodegenerative disorders
Given CDK5’s effect on neuronal tissue, it has been implicated in several neuronal pathologies of Alzheimer’s disease, amyotrophic lateral sclerosis (ALS), and Parkinson’s disease. Tau pathway has been implicated in diseases, such as Alzheimer’s where Tau hyperphosphorylation is one of the main pathology and CDK5 is abnormally activated.
CDK5 dysregulation leads to hyperphosphorylation of Tau, which in turn contributes to the formation of neurofibrillary tangles. The abnormal phosphorylation of Tau and its accumulation is associated with Alzheimer’s disease.
Apoptosis
CDK5/Tau induced apoptosis is caused by the build-up of Tau. Tau accumulation disrupts the clearance of ubiquitinated proteins, which leads to accumulation of unfolded proteins inside the ER. This in turn impairs the ERAD pathway, activating the unfolded protein response which leads to apoptosis of neurons.
Abnormal phosphorylation by CDK5 in pathological states has been hypothesized to be due to an increase in the rate of phosphorylation at certain sites. There have been indications that phosphorylation of Tau in neurons increases upon exposure to Aβ, hypoxia, oxidative stress, inflammation, and excitotoxicity. Aging has also been suggested to be a stress factor in promoting increased phosphorylation.
Recreational drugs
The CDK5/Tau pathway has been implicated in recreational drugs. Exposure to methamphetamine leads to upregulation of CDK5, leading to increased phosphorylation of Tau. It has been hypothesized that it is through this pathway that methamphetamine causes neuronal apoptosis via Tau accumulation mediated impairment of ERAD. This, along with certain other factors, may underlie the apparent pathological similarities between methamphetamine abusers and neurogenerative disease patients.
CDK5 is believed to have an active role in contributing to several disease phenotypes. Thus, research on ways to regulate CDK5 activity are thought to alleviate or remove disease symptoms. Several small molecule CDK5 inhibitors have been found, inhibiting CDK5 by competing with ATP or Tau binding.
Further Reading
Last Updated: Jan 14, 2019