Skip to
Telomeres are crucial parts of the chromosome that act to protect them and ensure DNA replication is performed effectively. Mutations and defects with telomeres can cause a multitude of health complications. However, researchers aim to use telomeres as therapeutic targets for the treatment of some of these complications.
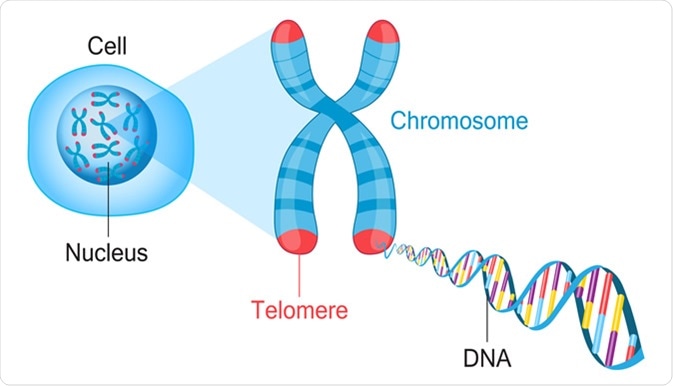
Telomeres are protective caps on the end of chromosomes. Image Credit: Fancy Tapis / Shutterstock
DNA replication
DNA replication is the most fundamental process in the cell cycle of all living organisms. It is responsible for producing two copies of the genome prior to cell division, which ensures new cells can be made.
DNA replication begins at certain points within the genome called DNA replication origins. In eukaryotes, this replication origin contains a binding site for the origin recognition complex (ORC) which starts the process of DNA replication.
The binding of certain proteins to the ORC facilitates further protein assembly to initiate the formation of the replication fork. The replication fork begins to unwind the DNA which allows DNA polymerases to bind to the leading strand. DNA polymerase continuously synthesizes new DNA along this strand as it is unwound.
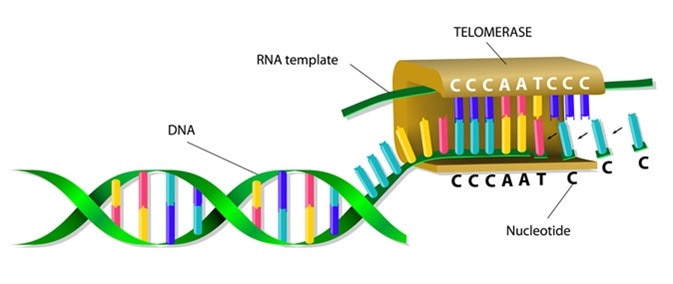
Telomerase elongates telomere. Image Credit: Designua / Shutterstock
As DNA polymerases only act in a 5’ to 3’ direction along the DNA, the lagging strand is not replicated the same way as the leading strand. Therefore, for the lagging strand, short RNA primers are attached to the DNA which can bind to DNA polymerases.
These primers are extended into Okazaki fragments. DNA replication along the lagging strand occurs in sections of Okazaki fragments that are joined together by DNA ligases. The process of DNA replication occurs on both strands of the unwound DNA until two whole copies are made. Following this, the rest of the cell cycle begins which ensure the two copies are separated into two individual daughter cells.
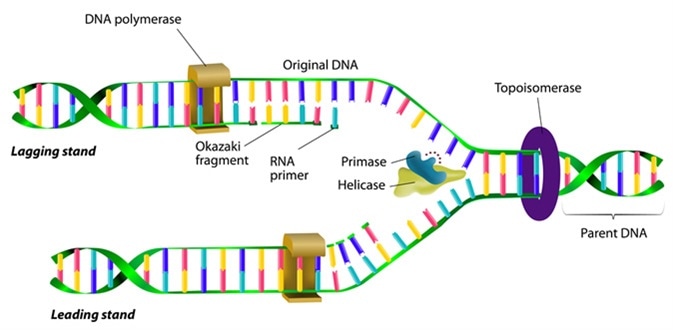
DNA replication. Image Credit: Designua / Shutterstock
Many DNA replication origins exist in the DNA; therefore, replication occurs at many points simultaneously. Replication forks meet and terminate at many points in the chromosome. DNA replication is unable to reach the end of the chromosome due to the linear nature of chromosomes. As a result of this, DNA is lost with each replication.
Telomeres are repetitive regions of DNA at the end of the chromosome that prevents the loss of genes due to the shortening of chromosomes. However, telomeres also naturally shorten with each set of DNA replication.
Telomere shortening
The role of telomeres is to protect the end of the chromosome from deteriorating or fusing with other near-by chromosomes. As explained above the telomeres shorten; giving them a set amount of times they can shorten before the cell can no longer undergo any more divisions. This is referred to as the “Hayflick limit”.
The shortening of telomeres is directly linked to the aging of living organisms. Telomere shortening can also cause other health complications, including cardiovascular and neurological conditions. Research has shown that telomeres play a role in the development and progression of cardiomyopathies in humans.
Results from a recent study concluded that heart failure patients encounter an increased amount of telomere shortening in their cardiomyocytes compared to individuals that do not have heart failure. The reason for this is not well understood as cardiomyocytes are post-mitotic cells that do not undergo replications.
Cell senescence is the process that disables the ability for a cell to proliferate, which is usually irreversible. Senescent cells have a particular set of characteristics that can be used to distinguish them from non-senescent cells. Telomere shortening can be used as a marker for cell senescence, which is the basis of the telomere aging theory. After a certain number of replications, the Hayflick limit of telomere shortening is reached, which leads to cell cycle arrest, cell senescence, and apoptosis. This occurs to protect against the consequences of telomere dysfunction.
Telomere research
Telomerase is the enzyme responsible for lengthening telomeres. This increases the time it takes for the chromosomes of a cell to reach the Hayflick limit. Due to this, telomerase activity affects cellular aging and is linked to the development of cancer.
Recent studies have aimed to evaluate the potential for telomerase to act as a therapeutic target to reduce the effect of telomere shortening and reduce the risk of certain cancers. Telomerase can possess oncogenic properties; illustrated by the associations between the development of certain cancers (I.e. urothelial carcinomas) and mutations in the telomerase promoter.
These mutations can lead to abnormally high levels of the telomerase catalytic subunit; telomerase reverse transcriptase (TERT). Due to these properties, pharmacologically inhibiting telomerase could be a therapeutic option against certain cancers. Inhibiting telomerase can be done by:
- Directly targeting the hTERT component of telomerase
- Directly targeting the TERC component of telomerase
- Using immunotherapies to target telomerase
- Unintended off-target side effects from chemotherapy that targets telomerase
Manipulating telomerase may also be used to improve the symptoms of certain cardiovascular diseases. Senescence of various cardiovascular cells, in particular, endothelial cells (ECs) and vascular smooth muscle cells (VSMCs), has a role in the development of vascular lesions, resulting in the development of atherosclerotic plaques.
Studies have shown that the regulation of telomerase can reduce and even reverse the senescence of vascular cells which can restore their function. The activation of TERT reduces the proliferation and hypoxia of VSMCs, it also increases antioxidants and the anti-senescent activity in ECs. All of these changes lead to a reduction of atherosclerosis.
Sources
- Riera, A., et al. (2017). From structure to mechanism—understanding initiation of DNA replication. Genes & Development. https://dx.doi.org/10.1101%2Fgad.298232.117
- Leman, A. and Noguchi, E. (2013). The Replication Fork: Understanding the Eukaryotic Replication Machinery and the Challenges to Genome Duplication. Genes. https://dx.doi.org/10.3390%2Fgenes4010001
- Bernadotte, A., et al. (2016). Markers of cellular senescence. Telomere shortening as a marker of cellular senescence. Aging. https://dx.doi.org/10.18632%2Faging.100871
- Sharifi‐Sanjani, M., et al. (2017). Cardiomyocyte‐Specific Telomere Shortening is a Distinct Signature of Heart Failure in Humans. Journal of the American Heart Association. https://dx.doi.org/10.1161%2FJAHA.116.005086
- Ait-Aissa, K., et al. (2016). Friend or foe? Telomerase as a pharmacological target in cancer and cardiovascular disease. Pharmacological Research. https://dx.doi.org/10.1016%2Fj.phrs.2016.07.003
Further Reading
Last Updated: May 22, 2019