Electrophoretic mobility shift assay, sometimes referred to as EMSA, is a sensitive method for investigating nucleic acid-protein binding. This is based on gel electrophoresis and is based on the difference in the way nucleic acid-protein complexes and free nucleic acids move during gel electrophoresis or the difference in electrophoretic mobility.
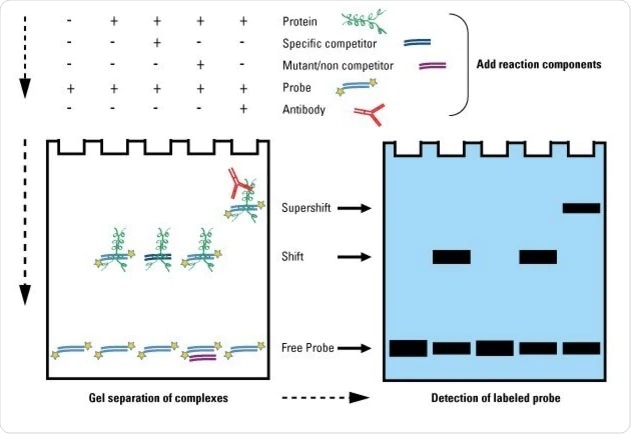
Image Credit: https://www.thermofisher.com/uk/en/home/life-science/protein-biology/protein-biology-learning-center/protein-biology-resource-library/pierce-protein-methods/gel-shift-assays-emsa.html
There are many protocols for electrophoretic mobility shift assays, with one involving using two-color fluorescence being used to visualize not only the nucleic acids but also the protein.
How does electrophoretic mobility shift assay work?
This is an example of an electrophoretic mobility shift assay using radioactively labeled DNA.
A 32P-labeled fragment of DNA containing the potential protein binding site is mixed with the protein of interest in a buffer system, consisting of HEPES-KOH, glycerol, EDTA, KCl, phenylmethylsulfonyl fluoride, and DTT. Once incubated, this is directly loaded into the gel.
Typically, a non-denaturing polyacrylamide gel is used and this needs to be “pre-run” beforehand. The gel can at this stage be fixed using a solution of methanol and acetic acid, which is optional. The gel is then dried before being exposed to autoradiography film.
The binding of the protein makes the nucleic acid-protein complex move slower than free nucleic acids, thus once the gel has visualized the difference in band location indicates whether the protein of interest has bound to the potential target DNA.
It is not always necessary to use radioactively labeled nucleotides; fluorophores can be used, so that fluorescence becomes the method of visualization. Another example is the use of biotin, which can be detected by either chemiluminescence or chromophore deposition. Alternatively, fluorophores or chromophores can be added after the electrophoresis, meaning that the target nucleic acid does not need to be labeled.
Jing and co. developed a fluorescence-based electrophoretic mobility shift assay that used fluorophores to not only visualize the nucleic acids but also the protein as well. This was achieved by using two fluorophores, SYBR Green for the nucleic acids and SYPRO Ruby for the proteins, which results in green and red fluorescence.
To validate the assay, the authors used a 40 nucleotides fragment from the lac operon operator alongside the lac repressor protein at varying concentrations. After incubation, these samples were then put into a 6% non-denaturing polyacrylamide gel. Once the electrophoresis was completed, the gel was stained with SYBR Green EMSA stain and visualized.
Following this, the gel was further stained with SYPRO Ruby EMSA stain and visualized again. The images can be overlaid together so that both fluorescent stains can be visualized at the same time.
The resulting gel showed that when the lac repressor binds to the target DNA sequence in the lac operon operator, this results in yellow color in the overlay image. The intensity of these bands’ changes with the concentration of the lac repressor protein, which is also seen with the SYPRO Ruby staining.
Therefore, this two-color fluorescence electrophoretic mobility shift assay is capable of detecting both nucleotides and protein in an electrophoretic mobility shift assay.
What are the advantages and limitations of electrophoretic mobility shift assays?
The advantages of electrophoretic mobility shift assay include simplicity, ability to be performed in a wide range of conditions, and high sensitivity. This means that electrophoretic mobility shift assays are simple to perform, where the assay conditions can be modified within a wide range to suit may nucleic acid-protein interactions; the temperature range is between 0˚C and 60˚C, the pH range between 4 and 9.5, and salt concentrations can range from 1mM to 300nM to give some examples.
The high sensitivity means that the concentrations of the nucleic acids and proteins can be lower than 0.1nM, and as it is an electrophoresis-based assay the sample volume can be less than 20μl.
While there are advantages, there are also limitations; these include the possibility of nucleotide-protein complex dissociation during electrophoresis, the possibility that the electrophoretic mobility of the nucleotide-protein complex is not only influenced by its size, and the inability of electrophoretic mobility shift assay to detect specific nucleotide sequences to which the protein binds.
What considerations are needed when performing an electrophoretic mobility shift assay?
While a standardized protocol may be sufficient for some electrophoretic mobility shift assays, some considerations need to be taken
Selecting the target nucleic acid
Short fragments of DNA or RNA are cheap and quick to obtain and make it less likely for non-specific binding to occur. This is particularly advantageous if the protein of interest has a low specificity towards its target nucleic acid.
However, care needs to be taken to ensure that binding sites are not too close to the ends, as this can lead to structural and electrostatic end effects, ultimately resulting in aberrant binding during electrophoretic mobility shift assays.
Competing proteins
Nucleic acid sequences can have more than one protein that binds to it, which is often the case. This may not be a problem, but sometimes the reaction of interest can be masked by the action of a secondary protein. In this case, unlabeled nucleic acids can be added to compete with the labeled nucleotides. For this to be successful, the secondary protein needs to bind both nucleotides equally well, while the target protein needs to have a higher affinity for the labeled nucleotides.
Binding conditions
Salt concentrations can impact protein-nucleic acid binding; therefore, this needs to be adjusted for the specific nucleic acid-protein complex before the electrophoretic mobility shift assay for the assay to be optimal.
The ability for electrophoretic mobility shift assays to be performed at a wide range of salt concentration should enable many nucleic acid-protein complexes to be analyzed by electrophoretic mobility shift assays.
Sources:
- (2005) Electrophoretic mobility shift assays Nature Methods https://doi.org/10.1038/nmeth0705-557
- Hellman, L. M., and Fried, M. G. (2007) Electrophoretic Mobility Shift Assay (EMSA) for Detecting Protein-Nucleic Acid Interactions Nat Protoc doi: 10.1038/nprot.2007.249
- Jing, D., et al. (2004) The utility of a two-color fluorescence electrophoretic mobility shift assay procedure for the analysis of DNA replication complexes Electrophoresis https://doi.org/10.1002/elps.200405994
Last Updated: Sep 29, 2020