The importance of in vitro transcription as a component of the mRNA therapeutic manufacturing workflow has grown significantly in recent years. The in-process raw materials for synthetic mRNA manufacturing must fulfill the necessary quality standards, function consistently, and scale up readily for commercial production to guarantee the safety and efficacy of the finished therapeutic products.
Promega offers cGMP and suitable raw materials to assist each stage of the development of mRNA therapeutics, regardless of the application, including immunotherapies, protein replacement, and vaccinations.
Quality
cGMP reagents for mRNA manufacturing
Trusted technology, modern modalities
For more than 40 years, Promega has been a top supplier of in vitro transcription reagents for a wide range of applications.
Promega has improved the manufacturing and quality attributes of its reliable raw material reagents, ensuring that they meet the stringent quality requirements for the manufacture of mRNA therapeutics to better serve and support the needs of customers in the quickly expanding field of mRNA therapeutic development.

Image Credit: Promega Corporation
Primary manufacturer
Promega oversees every facet of product manufacturing as a primary manufacturer, from raw materials to the final product. It successfully removes uncertainty to guarantee a steady supply with numerous global inventory sites and integrated logistics assistance.

Image Credit: Promega Corporation
Fit-for-purpose
Fit-for-purpose and cGMP reagents to take users from template to transcript
The most popular technique for achieving straightforward, scalable, cell-free mRNA synthesis is in vitro transcription-based manufacturing, which consists of the six major processes shown below.
Between its custom capabilities and product offerings, Promega has a solution for any mRNA synthesis need.
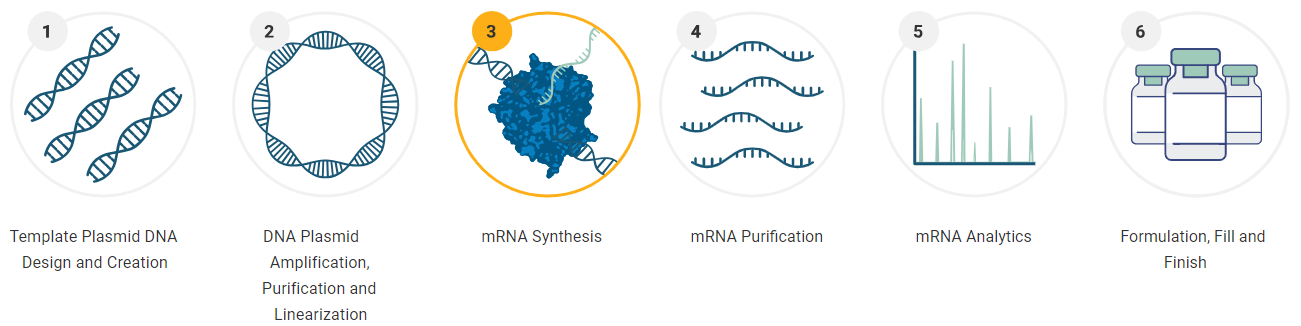
Image Credit: Promega Corporation
cGMP: More than a marketing term
In vitro transcription reagents produced under cGMP adhere to pertinent ICH Q7 GMP guidelines and are backed by an extensive quality control system.

Image Credit: Promega Corporation
Same manufacturing quality, additional testing if and when users need it
Users can upgrade to the cGMP products as they move from research into the clinical phases of development to obtain the testing and documentation needed to fulfill the quality requirements.
Source: Promega Corporation
| Research Use |
|
cGMP for mRNA Therapeutics |
 |
Function/Activity |
 |
| |
Animal Origin Free |
 |
| |
Contamination Testing For: |
|
 |
Nucleases |
 |
| |
Bioburden |
 |
| |
Host Cell DNA |
 |
| |
Endotoxin |
 |
| |
Documentation Available Upon Request: |
|
 |
Certificate of Analysis |
 |
 |
Certificate of Origin |
 |
| |
TSE/BSE Statement |
 |
Animal origin-free production
Raw materials and manufacturing techniques are free of animal origin.
Contamination testing
Lowers the danger of impurities that can affect the in vitro transcription product’s production and quality.
Quality documentation
On request, TSE/BSE statements, a certificate of analysis, and a certificate of origin are available.
Technical specification
cGMP-Manufactured In Vitro Transcription Reagent Specifications. Source: Promega Corporation
Custom capabilities
Wherever the user is in the process, Promega meets the user there
Promega provides raw materials for mRNA synthesis that bridge the gap between early discovery and commercialization, enabling it to grow with users as their manufacturing needs evolve and increase while also meeting users where they are in the research phase.
To provide precisely what the users require, Promega offers customized formulations, formats, and scales for both cGMP-grade and research-use reagents.
Partnerships
Small enough to listen, big enough to deliver
The field of mRNA therapies is quickly developing, with ever-evolving demands. To expedite their time to market, users want an innovative partner that can swiftly pivot, optimize their process, and scale production as manufacturing demands expand.
Users who work with Promega receive more than just scientific know-how—they also receive the support of a collaborative, seasoned workforce that cares about their success.

Image Credit: Promega Corporation
Let’s discuss your product requirements!