Mar 29 2004
Johns Hopkins University researchers have created a new class of artificial proteins that can assemble themselves into a gel and encourage the growth of selected cell types. This biomaterial, which can be tailored to send different biological signals to cells, is expected to help scientists who are developing new ways to repair injured or diseased body parts.
 "We're trying to give an important new tool to tissue engineers to help them do their work more quickly and efficiently," said James L. Harden (pictured at right), whose lab team developed the new biomaterial. "We're the first to produce a self-assembling protein gel that can present several different biological signals to stimulate the growth of cells."
"We're trying to give an important new tool to tissue engineers to help them do their work more quickly and efficiently," said James L. Harden (pictured at right), whose lab team developed the new biomaterial. "We're the first to produce a self-assembling protein gel that can present several different biological signals to stimulate the growth of cells."
Harden, an assistant professor in the Department of Chemical and Biomolecular Engineering, reported on his work March 28 in Anaheim, Calif., at the 227th national meeting of the American Chemical Society. His department is within the Whiting School of Engineering at Johns Hopkins.
Tissue engineers use hydrogels, which are macromolecular networks immersed in an aqueous environment, to provide a framework or scaffold upon which to grow cells. These scientists hope to advance their techniques to the point where they can treat medical ailments by growing replacement cartilage, bones, organs and other tissue in the lab or within a human body.
 |
James Harden, right, and doctoral student Stephen Fischer use confocal fluorescence microscopy to study how cells respond to their new protein hydrogels.
Photo by Will Kirk |
The Harden lab's new hydrogel is made by mixing specially designed modular proteins in a buffered water solution. Each protein consists of a flexible central coil, containing a bioactive sequence and flanked by helical associating modules on each end. These end-modules come in three distinct types, which are designed to attract each other and form three-member bundles. This bundling leads to the formation of a regular network structure of proteins with three-member junctions linked together by the flexible coil modules. In this way, the new biomaterial assembles itself spontaneously when the protein elements are added to the solution.
The assembly process involves three different "sticky" ends. But between any two ends, Harden can insert one or more bioactive sequences, drawing from a large collection of known sequences. Once the gel has formed, each central bioactive module is capable of presenting a specific biological signal to the tissue engineer's target cells. Certain signals are needed to encourage the adhesion, proliferation and differentiation of cells in order to form particular types of tissue.
Harden's goal is to provide a large combinatorial "library" of these genetically engineered proteins. A tissue engineer could then draw from this collection to create a hydrogel for a particular purpose. "We want to let the end-user mix and match the modules to produce different types of hydrogels for selected cell and tissue engineering projects," he said.
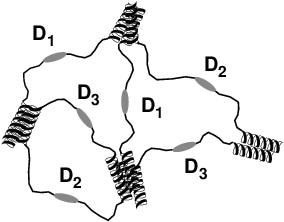 |
This schematic depicts the Harden team's hydrogel network with three distinct bioactive domains formed by the self-assembly of modular proteins.
Photo by Will Kirk |
Harden believes this technique may speed up progress in the tissue engineering field. For one thing, tissue engineers would not have to do complex chemistry work to prepare a hydrogel for each specific application; his hydrogels form spontaneously upon mixing with water. Also, unlike hydrogels that are made from synthetic polymers, the Harden team's hydrogels are made of amino acids, the native building blocks of all proteins within the body. Finally, more than one protein signaling segment can be included in the Harden team's hydrogel mix, allowing a tissue engineer to send multiple signals to the target cells, thereby supporting the simultaneous growth of several types of cells within one tissue.
"Our philosophy is to take a minimalist approach," Harden said. "Our hydrogels are designed to send only the growth signals that are needed for a particular application."
 Harden's colleagues in the hydrogel research are Lixin Mi, who earned his doctorate at Johns Hopkins and now is a postdoctoral researcher at the National Institutes of Health; and Stephen Fischer (pictured at right), a current doctoral student in the Department of Chemical and Biomolecular Engineering.
Harden's colleagues in the hydrogel research are Lixin Mi, who earned his doctorate at Johns Hopkins and now is a postdoctoral researcher at the National Institutes of Health; and Stephen Fischer (pictured at right), a current doctoral student in the Department of Chemical and Biomolecular Engineering.
The Harden team's research was supported by a grant from NASA through the Program on Human Exploration and Development of Space. Stephen Fischer is also supported by a NASA Graduate Student Researchers Program fellowship.