May 22 2009
In just two weeks from the time the first patient virus samples were made available, Singapore scientists report an evolutionary analysis of a critical protein produced by the 2009 H1N1 influenza A virus strain.
In the Biology Direct journal's May 20th issue, Sebastian Maurer-Stroh, Ph.D., and his team of scientists at the Bioinformatics Institute (BII), one of the research institutes at Singapore's Biopolis, also demonstrated the use of a computational 3-dimensional (3D) structural model of the protein, neuraminidase.
"Because we were working as a team, driven by the common goal to understand potential risks from this new virus, our group at BII was able to successfully complete this difficult analysis within such a short time," said Dr. Maurer-Stroh, BII principal investigator and first author of the paper.
BII's interactive 3D model is available at the following link: http://mendel.bii.a-star.edu.sg/SEQUENCES/H1N1/
With the 3D model, Dr. Maurer-Stroh and his team were able to map the regions of the protein that have mutated and determine whether drugs and vaccines that target specific areas of the protein were effective. Among their findings:
a. neuraminidase structure of the 2009 H1N1 influenza A virus has undergone extensive surface mutations compared to closely related strains such as the H5N1 avian flu virus or other H1N1 strains including the 1918 Spanish flu;
b. neuraminidase of the 2009 H1N1 influenza A virus strain is more similar to the H5N1 avian flu than to the historic 1918 H1N1 strain (Spanish flu);
c. current mutations of the virus have rendered previous flu vaccinations directed against neuraminidase less effective; and
d. commercial drugs, namely Tamiflu and Relenza, are still effective in treating the current H1N1 virus.
With the Biology Direct journal paper, the Singapore scientists have become the first to demonstrate how bioinformatics and computational biology can contribute towards managing the H1N1 influenza A virus.
"BII's H1N1 virus sequence study marks a significant milestone in the use of computational biology methods in understanding how the mutations of the fast evolving influenza virus affect immunogenic properties or drug response," said BII Director Frank Eisenhaber, Ph.D. "This information helps to develop a strategy for fighting the H1N1 virus and for organising an effective treatment for patients."
Other technologies to tackle the 2009 H1N1 Influenza A virus have been developed by scientists at Biopolis research institutes sponsored by Singapore's A*STAR (Agency for Science, Technology and Research). They include:
- a chip that is able to quickly sequence or decode the genes in the flu virus and distinguish between the H1N1, seasonal, and mutated flu strains, at the Genome Institute of Singapore (GIS).
- a microkit for the detection and identification of the flu virus strain within 2 hours, at the Institute of Bioengineering and Nanotechnology (IBN).
- a molecular diagnostic assay to distinguish between the H1N1 and seasonal flu strains, at the Institute of Molecular and Cell Biology (IMCB).
The Singapore scientists' paper, "Mapping the sequence mutations of the 2009 H1N1 influenza A virus neuraminidase relative to drug and antibody binding sites," was published in Biology Direct journal on 20 May 2009. Authors: Sebastian Maurer-Stroh1, Jianmin Ma1, Raphael Tze Chuen Lee1, Fernanda L Sirota1 and Frank Eisenhaber1,2 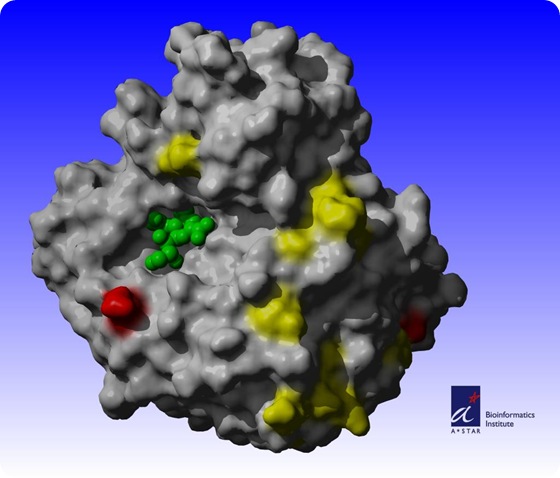
1Biomolecular Function Discovery Division, Bioinformatics Institute (BII), Agency for Science, Technology and Research (A*STAR), Singapore 2Department of Biological Sciences, National University of Singapore, Singapore
Influenza A virus strains are categorized according to two proteins found on the surface of the virus: haemagglutinin (H) and neuraminidase (N). All influenza A viruses contain haemagglutinin and neuraminidase. The structures of these proteins differ from strain to strain eg, swine flu belongs to the H1N1 type, avian flu to H5N1 and the currently dominant seasonal flu belongs to the H3N2 type.