Apr 12 2010
A supplier of tools to aid in speech therapy has switched from conventional thermoplastic elastomers to Medalist® medical elastomers from Teknor Apex Company, citing advantages in compound selection, blending, molding, and resistance to chewing and other rugged treatment.
ARK Therapeutic Services, Inc., owned by speech-language pathologist Debra C. Lowsky, MS, CCC-SLP, and her husband John Lowsky, a mechanical engineer, is a company that designs, manufactures, and distributes speech and occupational therapy tools and other healthcare products. Many of its devices are for use only by professional therapists or trained caregivers to help correct sensory oral motor problems by stimulating muscles that correlate with specific movements of the lips, tongue, jaw, etc. ARK Therapeutic Services now uses Medalist elastomers for the soft tips of oral probes, a complete line of chew tools, and the Z-Vibe® vibratory tool. Non-speech applications of Medalist elastomers include lids for the Sip-Tip® liquid feeding cup and the E-Z Eye Med® dispenser for eye medicine.
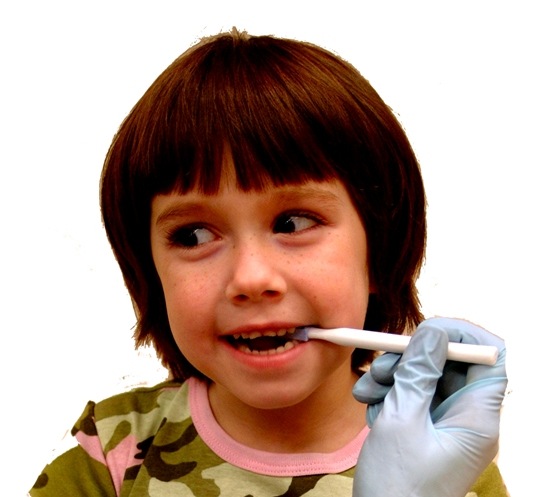
The company’s switch to Medalist compounds was prompted by its plan to obtain the CE mark, which requires subjecting each raw material to costly and stringent biocompatibility tests to meet European Union standards. “Every grade in the Medalist range of compounds has already been pre-tested for cytotoxicity, one of the three biocompatibility categories required,” said Lowsky. “In addition, we found that we could use four different Medalist compounds to produce the same number of applications that previously required eight or nine grades of the other elastomers. The Medalist compounds blend very readily, enabling us to vary durometer without increasing the number of materials in our inventory.”
The Medalist compounds are also easier to process, noted Lowsky. Cycle times are shorter, and there is no need for processing aids, additive lubricants, or silicone releases.
One unlooked-for advantage of the Medalist compounds was their superiority in the special type of toughness required for oral tools to endure during repetitive biting and chewing exercises required to improve feeding skills. “We built our own machine to test what we call ‘chewing cycle durability’ by simulating the biting of one of our oral therapy tools at different frequencies—for example, one chew per second,” said Lowsky. “While a tool made from a well-known standard brand of elastomer began to break down after 80,000 cycles, the same product molded from a Medalist compound withstood 120,000.”
Recently ARK Therapeutic Services began redesigning its Z-Vibe tool to make it a one-piece product of all-elastomer construction. Until now this has been a two-part product, with an elastomeric tip and a polypropylene handle. Inside the handle is a tiny battery-powered motor that provides the vibration.
ARK Therapeutic Services began switching to Medalist compounds six months ago. Lowsky credits Teknor Apex for its technical support and responsiveness, including speed in providing samples for product development. The company uses three different grades from the Medalist Versatile Series, with Shore A durometer ranging from 43 to 87. As with all materials that ARK Therapeutic Services uses for oral tools, Medalist compounds are compliant with regulations of the U.S. Food & Drug Administration and are latex- and phthalate-free.
www.arktherapeutic.com