By inputting several parameters, the clinician can customize how the device will respond to each individual patient and can gradually adjust those parameters as the patient progresses. The device itself is attached to the patient’s affected leg and connected to a foot sensor that fits inside the shoe.
The foot sensor detects when weight is applied, which triggers mechanical assistance, helping the patient stand, walk and ascend or descend stairs. Unlike other robotic devices, the bionic leg will only activate if the patient intends on moving and will do nothing if they don’t. This intention to move has been shown to be a key ingredient in active motor learning, also called neuroplasticity, and is showing very promising results both on acute as well as chronic patients undergoing rehabilitation.
How did the Bionic Leg originate?
After experiencing a knee injury while playing sandlot football in high school, Tibion founder, Bob Horst wasn't satisfied with the doctor's solution for keeping him mobile — crutches. "That seemed primitive to me," he said. And so started Bob’s dream to create a better solution.
In 2002, he co-founded Tibion Bionic Technologies with a mission statement that satisfied his high school hope: "Advancing rehabilitation and mobility with innovative technology."
Despite years of uncertainty about whether a noninvasive, computerized assistive device could have therapeutic value, the Bionic Leg Orthosis was born. "We are the first to have a portable device like this," Horst said. "What really makes ours effective is that it is activated by the patient’s intent. … It is unlike many robotic therapy devices in that it does not have a set of movement patterns or speeds, but continually reacts to the intended motion of the patient. This is a key reason the Bionic Leg is showing so much promise in rehabilitation."
Tibion began shipping the Bionic Leg to clinics at the end of 2009, and have since treated hundreds of patients. Each physical therapy session with the Bionic Leg lasts about an hour, with patients performing simple repetitions like going up and down a step, sitting and standing, walking, and balancing. The technology has allowed mobility impaired patients to regain mobility they may have been told was irreversibly lost.
"Our goal is to become the standard of care for stroke rehabilitation. Most stroke patients get no more therapy after the first six months because it's not effective. But we've seen patients five or 10 years after a stroke who are noticeably better after using this technology."
How has the Bionic Leg developed since it was first invented?
Since the original prototype, several ongoing improvements have been made to the leg. Most noticeably would be additional refinement of the internal motors, making the operation smoother and more sensitive. Improvements to the textiles making them more comfortable and better fitting have been made as well as improvements to the reel and lace tightening system. Changes to the rigid structure have also been made making the device lighter and stronger, improving its overall function and durability.
What is new about Tibion’s Bionic Leg?
The most recent development is the addition of wireless reporting capabilities of the functional measures that the leg is capturing. The current version of the device counts various parameters such as the number of steps taken, sit-to-stand cycles and the amount of time the device is on. This information can be accessed through the input panel, however it is currently in the form of raw data.
This new version of the device will take the raw data and convert it into meaningful functional measurements specific to that patient, and send it back to the facility in the form of a patient session report.
This report will be useful as a documentation and progress tool that can be used by clinicians as well as administration. This is a very exciting development for the company as it will not only provide critical information relative to patient outcomes and their progress, but it will also represent a significant time savings for customer in obtaining it. As accountable care organizations continue to develop over the next several years, it will be critical to be able to provide data like this for clinical and business related purposes.
What types of patient is Tibion’s Bionic Leg aimed at?
Patients that are experiencing generalized lower extremity weakness and need to relearn how to use their affected limb. A moderate amount of trunk control and hip flexor strength are ideal. Typically neurological conditions such as stroke, multiple sclerosis, traumatic brain injury and partial spinal cord injuries are some of the more common indications, however various orthopedic, bariatric and even cardiac applications can also apply.
This is a device that helps to break down typical compensating behaviors that patients struggle with as they go through various forms of therapy and helps increase their confidence, desire to walk and ability to move under their own power.
How common are these conditions?
Unfortunately, very common: Approximately 800K strokes/year in the US with approximately 350K suffering from mobility impairment. There are about 400K Americans with multiple sclerosis and about 300K brain injuries each year resulting in hospitalization. About 250K Americans suffering from incomplete spinal cord injuries with about 10K added each year.
How is the Tibion Bionic Leg activated by the patient’s intent to move?
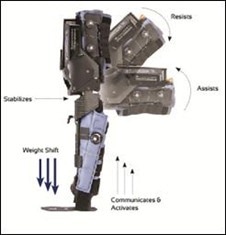 Foot sensors placed in the patient’s shoe detect the amount and distribution of the patient’s weight. This activates the device and provides mechanical assistance to the leg according to the therapist’s inputs.
Foot sensors placed in the patient’s shoe detect the amount and distribution of the patient’s weight. This activates the device and provides mechanical assistance to the leg according to the therapist’s inputs.
If the patient fails to put the required amount of weight into the foot sensor, the device will remain idle. Once the required amount of weight is reached, the device is activated and the therapy begins.
This design feature requires a greater level of participation from the patient, which has been shown to be a key element in relearning how to use the affected limb.
Will the Bionic Leg vary for patients with different conditions?
 The actual leg itself remains the same, however the settings can be adjusted depending on the level of function that the patient presents. There are 5 settings that the clinician will enter into the control panel of the device.
The actual leg itself remains the same, however the settings can be adjusted depending on the level of function that the patient presents. There are 5 settings that the clinician will enter into the control panel of the device.
- Weight: Patient’s weight in pounds or kilograms
- Threshold: The percentage of the patient’s body weight required to be on the foot sensor in order to activate the device.
- Assistance: This is the percentage of force applied by the device to help the patient perform knee extension
- Resistance: The amount of force applied by the device to limit the rate of knee flexion
- Extension Limit: Degrees from full extension to which the device will provide assistance
Each of these settings are adjustable and intended to be set specifically to each type of patient as well as to their level of function. For example, a lower functioning patient may need more assistance or resistance from the device and require a lower threshold in order to activate it, whereas a higher functioning patient may need less assistance and a higher threshold.
This is where the clinician plays a critical role in working with the device and the patient to ensure that the maximum benefit is being received. It is also important that the settings are being adjusted as the patient progresses so the device is assisting only when needed and backs off when it’s not.
How does the Bionic Leg fit with Tibion’s aims?

 Tibion’s mission is to create a new standard of care for mobility impairment therapy. The Bionic Leg fits that goal by providing a tool, that when used consistently and frequently supports the principles of neuroplasticity and motor learning which have been shown to be effective and lasting. It provides an extra set of hands for the clinician, allowing them to focus on the big picture when delivering therapy as well as reduces the need for additional resources that might otherwise be needed.
Tibion’s mission is to create a new standard of care for mobility impairment therapy. The Bionic Leg fits that goal by providing a tool, that when used consistently and frequently supports the principles of neuroplasticity and motor learning which have been shown to be effective and lasting. It provides an extra set of hands for the clinician, allowing them to focus on the big picture when delivering therapy as well as reduces the need for additional resources that might otherwise be needed.
Because it is wearable and portable, it can easily be incorporated with existing therapy concepts and equipment, allowing for more variety and creative applications during the therapy session.
Finally, the Bionic Leg protects its users. By stabilizing the knee joint, the leg helps to prevent the patient from falling, which can often result in injuries to themselves as well as their therapist. This added stability also helps to increase the patient’s confidence level and desire to walk – another key ingredient to the therapy process.
How do you see the future of the Bionic Leg developing?

 The Bionic Leg technology can be considered a platform for future product development. The potential exists to proliferate the current version of the Bionic Leg into products that could be used on other areas of the body such as the upper extremities, ankle, etc.
The Bionic Leg technology can be considered a platform for future product development. The potential exists to proliferate the current version of the Bionic Leg into products that could be used on other areas of the body such as the upper extremities, ankle, etc.
The current version of this product is a tool that is used in the clinic, however, the company sees a significant opportunity in the future to develop a device that could be used by the patient in the comfort of their own home. Doing this would allow patients to be able to extend their therapy experience in the clinic, and continue to benefit from what the Bionic Leg has to offer. Most likely this would require changes to the existing design, but it is believed to be real possibility as the company grows.
What are Tibion’s plans for the future?
Tibion’s near term goals are to drive this technology into the marketplace as quickly as possible by acquiring new users as well as growing the existing users. This will be accomplished by increasing the company’s sales and marketing presence, delivering the next generation products and by executing the company’s clinical research strategy.
Where can readers find more information?
Tibion Bionic Technologies
About Rob Cripe

Rob Cripe, Sr VP Marketing. Joining Tibion in Jan, 2012, Rob brings over 22 years of experience in the medical device arena with a proven track record worldwide commercial execution. His expertise in upstream and downstream marketing, product launch processes, portfolio planning, branding and messaging, medical education, and sales and distribution networks has been refined in large, medium and small companies throughout his career.
Starting in Warsaw, Indiana, the “Orthopedic capital of the World”, Rob has held executive positions with companies such as Biomet, Interpore Cross, Smith and Nephew, Pegasus Biologics, and most recently DJO Global where he served as the SVP of Strategic Marketing.
Throughout his career, he has adjusted his commercial execution strategies to maximize effectiveness for the organization and meet the customer’s needs.
Rob earned his undergraduate degrees in business and communications from Grace College in Warsaw, making his career in medical devices a natural fit.