
Please can you give a brief introduction to Cook Medical and Otolaryngology (ENT)?
Cook Medical is the world’s largest privately held medical device manufacturer. The ENT division is Cook’s 10th business unit/clinical division and we are excited to meet the needs of ENT patients. What makes Cook very unique and different in this ENT market is that it hasn’t been served by a manufacturer that has as broad manufacturing capabilities as we do.
There is a tremendous amount of clinical need because this specialty as a whole, ENT, has some very significant chronic disorders that can be both difficult to treat and challenging for the patient. I’ll give you two examples: obstructive sleep apnea and dysphagia.
Obstructive sleep apnea is a tremendous burden. In the US, there are about 25 million patients that suffer from obstructive sleep apnea, so it is an enormous problem.
Obstructive sleep apnea is not just a snoring problem, when you don’t get a good night’s sleep because you are continuously interrupted throughout the night, then it actually affects and can cause high blood pressure, diabetes and many other factors that are just starting to be known.
Research over the past 3-5 years has certainly shown that you need to aggressively do something more about obstructive sleep apnea.
Another area is dysphagia, or difficulty swallowing. Patients that are ageing, stroke patients and patients with head and neck cancer can have issues with swallowing. You and I may enjoy eating three meals a day. However, patients who survive their head and neck cancer may not be able to eat.
This is another huge area - around 20 million US patients suffer from some sort of difficulty swallowing. It is a huge social burden and a significant quality of life issue.
When we look at just those two areas we see that there is tremendous potential to innovate and create minimally invasive solutions to improve these patients’ lives.
How many patients are treated by ENTs and Otolaryngologists each year?
That is a difficult question. Just looking at the two examples that I’ve just provided, we can see that it is a very large patient population.
Chronic sinusitis is another tremendous issue in reducing work productivity and people missing work due to chronic sinus infections. In the US, there are between 350,000-500,000 endoscopic sinus surgical procedures performed each year – and that is just one procedure for one condition.
Tonsillectomy is one of the most commonly performed procedures and that procedure is performed about 1.3 million times per year in the US.
Ear tubes is another commonly performed procedure which happens about 900,000 – 1 million times a year.
So when you look at the overall larger patient populations of ENTs or Otolaryngologists that is a difficult one to answer. It is clear that we are talking very large volumes of procedures though.
Why do you think few companies have dedicated their efforts specifically for the needs of ENTs and Otolaryngologists?
I don’t think there have been few companies which have dedicated their efforts. There certainly have been some very large public manufacturers that have dedicated a tremendous amount of resources and efforts, but for the most part it has been focused on the surgical aspect.
Where Cook is unique is that we are bringing the minimally invasive solutions. Where we saw that there was a need was that there were surgical options, just as in any other specialty, but then there are ineffective medical treatments or pharmaceutical solutions – drugs.
You can give a patient nasal sprays all day long but that is not going to cure the infection. At the other end of the spectrum is surgery, so you go in and surgically remove all the tissue into the nose. Researchers have found that while that may help a percentage of that population, it doesn’t cure the disease and that may not be the gold standard to perform. Where there is a gap is in the middle - creating those minimally invasive solutions.
What problems can this lack of dedication to minimally invasive solutions cause?
It may cause a lack of innovative solutions, so patients may not benefit from these minimally invasive solutions.
If you look back many years ago into cardiology or heart surgery, the gold standard was performing open heart surgery. But then cardiac stents came along and revolutionized how patients were treated – instead of opening their whole entire chest to do a heart procedure, now they were treated with a minimally invasive device and therefore recovery time and complications were reduced. This is a very similar thing to what is happening for ENTs.
Companies have dedicated the resources, but where resources have been somewhat lacking is in the research area of ENT.
Looking at the example of cardiology again – if a patient has a heart condition that is not treated effectively then the patient may die. Most ENT disorders, except head and neck cancer, do not necessarily cause death.
Government-funded research usually goes to those diseases that have very high potential mortality and ENT has not been there so Government funded research has been lacking.
Please can you outline the new Otolaryngology, Head and Neck Surgery (OHNS) division recently launched by Cook Medical?
We launched the OHNS division from a product development perspective and it does take a couple of years to develop those products. Even though we launched it in August 2010, we have just commercially launched it externally just this year. So that began in early 2012 in the US and clearly we’ve seen the interest and the embracing by the community hence the reason we are now expanding into the UK and Germany.
What are the main aims of Cook Medical’s OHNS division?
Our main aims are to create those innovative minimally invasive solutions to help address some of these chronic problems so we can help improve patient outcomes.
Whenever we make product development decisions they are based upon the unmet clinical need and how can we improve the outcome of patients.
When we look at the very large patient population in the ENT world, especially these chronic disorders, we feel that there is a significant need to develop more solutions.
Please can outline the salivary duct access and stone remover set launched by Cook Medical?
These are other great examples of minimally invasive solutions. I will say that the salivary endoscopy procedure has been pioneered in Europe and in fact there are some London researchers that have been on the very cutting edge of developing this procedure. This is a prime example of surgical options going to more minimally invasive solutions.
Most people are unaware that your salivary glands can become obstructed or blocked, but most people are familiar with kidney stones. These types of stones can develop in your salivary ducts. Then when a patient tries to eat they experience lots of pain, swelling because their salivary gland is trying to push saliva out of the duct but the duct can be blocked.
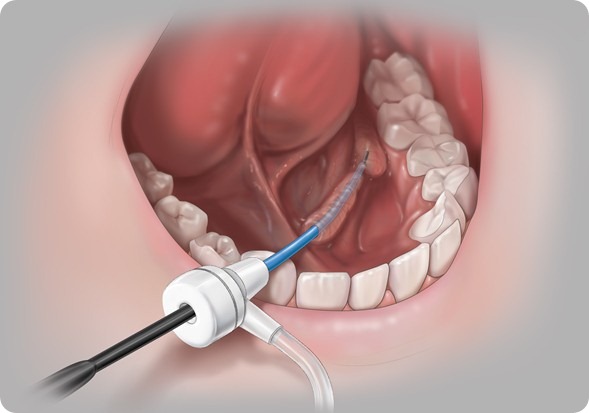
Previously, the only solution was to surgically remove the duct. Researchers, including the ones in London, have developed a technique where you can now go in to the salivary duct with minimally invasive access and a small endoscope to actually see the blockage and take it out.
This is what they have done in the urinary system for 20 years, but the technology wasn’t available for salivary endoscopy because the instruments weren’t small enough to actually access the salivary duct.
We are helping to develop more tools that allow you to access the salivary duct more easily and the stone retrieval set and basket help capture the stones and pull them out. So now the patient does not have to have their salivary duct removed.
Please can outline the ultrasound-guided thyroid biopsy needle launched by Cook Medical?
Thyroid nodules are very common as are thyroid cancers. There are about 40,000 thyroid cancers that are diagnosed every year in the US and an even greater percentage have regular thyroid nodules in their neck that need to be diagnosed to determine whether they have thyroid cancer.
What we are seeing now is that clinicians, head and neck surgeons, ENTs are using ultrasound to actually go in and biopsy those nodules to see if they are cancerous. This can be done in the clinic.
What our needle does is it now allows you the capability to visualize that needle. When you are using ultrasound you can visualize the exact pathway of the needle when it goes into the nodule, so this gives you a much greater accuracy to capture that biopsy.
What plans does Cook Medical’s OHNS division have for the future?
Our future is tremendously bright. We are excited about what we are doing and now as we are entering into the UK and German markets there is a tremendous opportunity to help meet patient needs within these markets but there is also certainly more need to continue looking for innovative solutions and to work with innovative researchers to develop these solutions.
Our future is certainly based upon those direct collaborations with researchers to develop those solutions. We are committed to this market and we are committed to these patients. We are a global organization and we will expand into other countries in Europe and eventually into the Asian markets as well.
Would you like to make any further comments?
I would just like to stress that because our product development decisions are based on improving patient care, we can be different. It is not necessarily the largest market but it is about improving patient care.
Salivary endoscopy is a prime example. Salivary stones aren’t as common as chronic sinusitis and they are not nearly as common as obstructive sleep apnea or other very common ENT disorders, but we saw that there is a tremendous clinical need.
If a patient has a stone that is in their salivary duct that is on their cheek and they have open surgery, they have a 35% chance for getting facial paralysis. We saw that need and we developed more tools for these surgeons to help perform this procedure. We want that chance for facial paralysis to be 0.
Our goal is to help those single solutions for both ENT surgeons and patients to help treat these chronic ENT disorders in a minimally invasive way. Our goal is to improve patient care.
Where can readers find more information?
www.cookmedical.com
About Thomas Cherry
 Thomas Cherry joined COOK Medical as District Sales Manager with the Critical Care Division in 1999.
Thomas Cherry joined COOK Medical as District Sales Manager with the Critical Care Division in 1999.
Mr. Cherry has held numerous management positions of increasing responsibility over the past 14-years, including Product Manager, Product Development Manager, Director, Business Development, and now Global Business Unit leader of COOKs newest Otolaryngology/Head & Neck Surgery division.
Mr. Cherry has extensive experience with global product launches and driving adoption of new technologies including minimally-invasive salivary introducers, antibiotic impregnated central venous catheters, self-advancing enteral feeding tubes, and balloon-assisted percutaneous tracheostomy products.
Prior to joining COOK, Mr. Cherry was clinically active for 6-years as a Critical Care Registered Nurse in cardiovascular, trauma, and surgical intensive care units.
Mr. Cherry holds a BSN from Southeastern Louisiana University and has also served 11-years in the United States Army National Guard as both an Officer in the Army Nurse Corps and Combat Flight Medical Specialist.
Mr. Cherry has been committed to advancing early-stage technologies into commercialization and education of the product development process while serving on the Advisory Board for the Northwestern University NUvention Medical Device Innovation Course and Fellowship program.
He has been an active member of Association of University Technology Managers (AUTM), the Society of Critical Care Medicine (SCCM), and Association of Professionals in Infection Control (APIC), while serving on various committees within each.