
What is obstructive salivary gland disease and who does it affect?
Everyone is familiar with urinary stones (kidney stones). The same thing can actually happen in your salivary ducts. These types of stones and strictures occur not nearly as frequently as kidney stones but they still occur quite often. The latest data we have seen showed about 1 in 5,000 patients actually have some type of obstructive salivary duct disorder.
Obstructive salivary gland disease can affect the very young all the way up to the elderly so it is not really a specific patient population. It occurs twice as often in males as it does in females, but it is very similar to kidney stones as well where sometimes it can just be hereditary. Also, in the patients affected, salivary stones, like kidney stones, tend to reoccur.
We all salivate throughout the day – all day long. Any time you put something in your mouth your salivary glands produce saliva so you can help chew your food. What happens in obstructive salivary disease is that every time a patient eats something, the side and/or floor of their mouth becomes very painful or even starts to swell.
This is because the duct (a long tube), which normally secretes saliva into the mouth, becomes blocked by either a stone or by the tightening of that tube. Consequently, the saliva can’t go anywhere. Thus every time you eat, the gland is trying to produce saliva but the saliva can’t go anywhere, this causes pain and swelling. This usually leads to infections as well.
What are the symptoms of obstructive salivary gland disease and how it is diagnosed?
It is usually diagnosed today when the patient experiences pain and swelling and goes to see their physician or local ENT physician. They will usually get some either X-rays or CT scans (CAT scans). These are the primary ways.
Some more advanced ways that we are seeing today is by using ultrasound guidance. This is used more in Europe than in the US at the moment. There are some select centers in North America that are using ultrasound to diagnose obstructive salivary gland disease as well.
How has obstructive salivary gland disease traditionally been treated?
Traditionally, the patients will be given medications. If they have an infection they will be given antibiotics. If it is swelling, they’ll be given medications, such as steroids, to decrease swelling. However, these medications don’t treat the underlying problem.
If the stone is small enough and they can feel it with their fingers, they even try to manually manipulate it out of your duct. This is not always successful – especially if the stone is large.
Predominantly the obstructive disease is caused by stones that are lodged in there, but you also have strictures which are tightenings of the tubes or the ducts. These have not been able to be treated in the past. But now using endoscopes they can see that it is a stricture. When they see that it is a stricture they’ll either put some medications in there or put some of our dilators in there to open up those strictures.
Historically it has been mostly a conservative approach because they didn’t want to go to the surgical approach because they knew of all the complications that can occur.
What happens if you don’t treat obstructive salivary gland disease?
If you don’t treat salivary gland disease it continues to swell and causes a major quality of life issue, consequently people don’t even want to go out. We had a conversation with one of our physicians in North America this week who has patients that won’t even go out to eat at dinner with friends because it is too painful and they are embarrassed.
What risks are involved with invasive open surgery to treat obstructive salivary gland disease?
There may be complications of surgically removing the salivary duct or the gland. When you are removing a salivary duct in your cheek you have the potential for hitting the very vital structures – particularly the nerves in your face. There are facial nerves that run all along your face and one of the common complications of this procedure is facial paralysis. There is a 35% chance of facial paralysis with traditional surgical techniques to treat obstructive salivary gland disease.
Some of the other risks would be that it can clearly also cause significant pain. You have these two major ducts that are in your cheeks but you also have two major ducts that are in your jaw – on the lower floor of your mouth. When they have to remove those they have to surgically open up the floor of your mouth, which is a highly sensitive area. So there is significant pain associated with that post surgery.
The three main complications that can occur with this surgery are:
- Facial paralysis
- Post-operative pain
- Potential airway complications caused by a lot of bleeding in your mouth
Please can you outline the suite of salivary duct access products that Cook Medical has recently launched that offer minimally invasive options for the treatment of obstructive salivary gland disease?
We are really excited about this as we feel that we can advance the minimally invasive procedure and give both clinicians and patients an option. One of the most challenging parts of performing a procedure that is minimally invasive with these very small endoscopes is actually accessing these very small salivary ducts. You can imagine how small these ducts are as we can’t see them with the naked eye. You actually have to use a microscope to see into the mouth and identify where these salivary ducts are located.
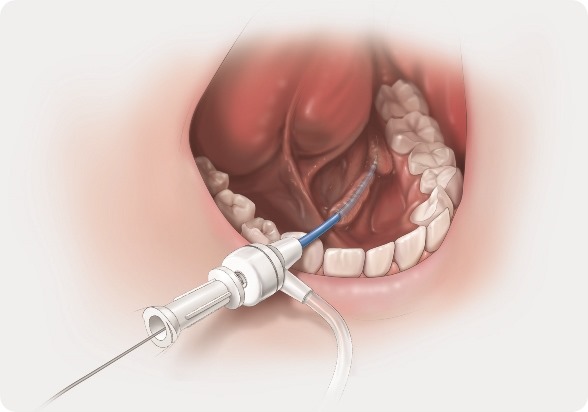
What our early tools do is allow you to more easily access those ducts. The small instrumentation with our guidewires helps us to get in and then we have our dilator set which allow us to open up the papilla (opening of the salivary duct) so it widens and opens up that area.
Then we have our Kolenda introducer set, which comes in after we have dilated the salivary duct, to put into the duct to maintain the access so we can pass the instrumentation in the duct during the entire procedure. Maintaining access is vital and can greatly reduce procedural time. Those are some of our early products.
We also have our nitinol extractor baskets which allow us, once we’ve accessed the duct and maintained access using the introducer, along with the endoscopes to go in and take out and retrieve the stones that are inside the ducts.
How were these devices developed?
These devices were developed in conjunction with working with experts here in North America. We worked particularly closely with one of the inventors of the introducer - Dr Jack Kolenda from Toronto, Canada. He was the first physician in North America to begin performing this procedure.
How do these products differ from others on the market?
There are numerous differences. It is a very young procedure – it was invented and pioneered in Europe, particularly in Germany because a company that is based in Germany that makes endoscopes made an endoscope that was small enough to go into these ducts. You can imagine the salivary ducts are very small – the largest size of these ducts is only about 5mm in diameter.
Even though it was pioneered in Europe this procedure has started to be performed over the past 4 or so years in North America. Where Cook comes in and where we differ is that we make the disposable devices that can help advance the procedure. We are very different to anything on the market.
In fact the only other tools that are available are the endoscope tools. They don’t have the tools that we are bringing to the market.
Is there a learning curve associated with using Cook Medical’s salivary duct access products?
Absolutely there is and that is where physician training comes into play. We are conducting physicians training throughout the United States. There is a training session upcoming at a medical conference in Orlando in the middle part of April and another at an university hospital in Chicago in mid-May.
What impact do you think Cook Medical’s salivary duct access products will have on the treatment of obstructive salivary gland disease?
I think we have the ability with not only these first introduction products that will start off by helping physicians more readily access these ducts, but I think that because in the pipeline we have several more products that are going to be released over the next 6months to 1 year, we have the ability to advance this procedure to the next level where more patients will be able to be treated.
Today there are limitations to the procedure. The limitations are that if there are stones that are larger than the salivary duct in North America there is no FDA approved product that is commercially available to help fragment those stones . In the kidney stone world there is shockwave lithotripsy which helps break up those stones, but that is only available in the urinary system in the kidneys, that is not available a in North America for the salivary glands.
So there are some limitations to the procedure but with the developments that we have in the pipeline we believe we are going to take this innovative approach to the next level.
Do you have plans to add further salivary duct access devices to this range?
We’re developing more interventional tools to help complete the procedure and broaden the patients that can be treated through more minimally-invasive salivary endoscopy. We are starting off with the accessing the duct, but where we are going next is the therapeutic interventional tools.
What are Cook Medical’s OHNS division’s plans for the future?
We are quite excited about the future, because this is just the start. We began commercialization with this new clinical division in March of last year; we are already expanding into the UK and Germany markets as we speak. We are going launching our new products in Europe during another conference at the end of April.
The future is certainly bright.We have a pipeline that is full to meet the unmet clinical needs in this patient population. We certainly expect to expand over the next 3-5 years and bring these devices and minimally invasive tools to help address these patients’ needs.
Cook is energized about this new clinical division and educating patients as well as clinicians about what we can do to help advance these procedures so that more patients can be served. We are here to improve patient outcomes. We are committed to the otolaryngology space and we look forward to
seeing what we can do to help this patient population.
Where can readers find more information?
About Thomas Cherry
 Thomas Cherry joined COOK Medical as District Sales Manager with the Critical Care Division in 1999.
Thomas Cherry joined COOK Medical as District Sales Manager with the Critical Care Division in 1999.
Mr. Cherry has held numerous management positions of increasing responsibility over the past 14-years, including Product Manager, Product Development Manager, Director, Business Development, and now Global Business Unit leader of COOKs newest division, Otolaryngology/Head & Neck Surgery.
Mr. Cherry has extensive experience with global product launches and driving adoption of new technologies including minimally-invasive salivary introducers, antibiotic impregnated central venous catheters, self-advancing enteral feeding tubes, and balloon-assisted percutaneous tracheostomy products.
Prior to joining COOK, Mr. Cherry was clinically active for 6-years as a Critical Care Registered Nurse in cardiovascular, trauma, and surgical intensive care units.
Mr. Cherry holds a BSN from Southeastern Louisiana University and has also served 11-years in the United States Army National Guard as both an Officer in the Army Nurse Corps and Combat Flight Medical Specialist.
Mr. Cherry has been committed to advancing early-stage technologies into commercialization and education of the product development process while serving on the Advisory Board for the Northwestern University NUvention Medical Device Innovation Course and Fellowship program.
He has been an active member of Association of University Technology Managers (AUTM), the Society of Critical Care Medicine (SCCM), and Association of Professionals in Infection Control (APIC), while serving on various committees within each.