Sartorius Stedim Biotech (SSB), a leading international equipment supplier for the pharma and biotech industries, has launched the new Sartocheck 4 plus Bag tester, the latest addition to its well-established Sartocheck line. The new system is the first device allowing reliable pre-use testing of single-use bioreactors after installation, based on the proven pressure decay measurement method. The Sartocheck 4 plus Bag tester directly responds to the market needs for increased security and quality assurance when single-use components are used. Designed for cell cultivation, single-use BIOSTAT CultiBag STR bioreactors with working volumes of 50 liters and 200 liters can now be conveniently tested by this new system. Later this year, the newly developed technology of this device will be extended to permit testing of 500-liter and 1,000-liter BIOSTAT CultiBag STR systems.
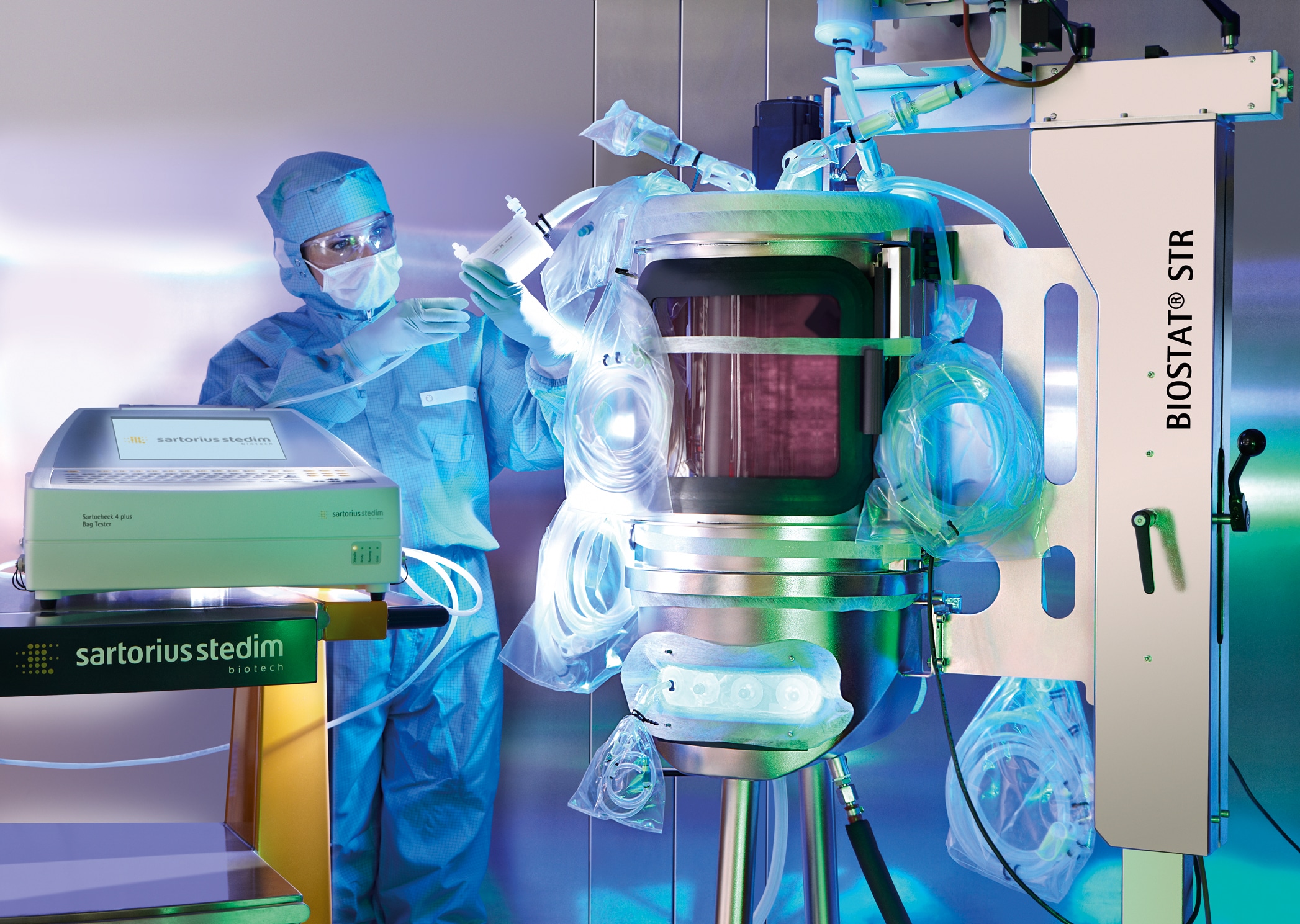 The Sartocheck 4 plus Bag tester performs a pre-use test of the entire bioreactor, including tubing. This device is capable of detecting typical leaks that might have been caused by operator handling errors upon installation of a bioreactor. Therefore, the bag tester is ideal for eliminating the risk of filling a defective single-use bioreactor with expensive cell cultures. The reason is its unique, patented fleece, which is inserted between the plastic film of the BIOSTAT CultiBag STR and the bag holding device of the tester. This fleece acts as a porous spacer, preventing direct contact between the bag and the smooth stainless steel surface of the holder. In addition, the fleece substantially reduces environmental heat transfer and, most importantly, eliminates the effects of masking of any potential pinholes. The high accuracy of the Sartocheck 4 plus Bag tester in measuring the pressure drop, along with its patented fleece technology, provides unrivaled reliability and reproducibility for testing.
The Sartocheck 4 plus Bag tester performs a pre-use test of the entire bioreactor, including tubing. This device is capable of detecting typical leaks that might have been caused by operator handling errors upon installation of a bioreactor. Therefore, the bag tester is ideal for eliminating the risk of filling a defective single-use bioreactor with expensive cell cultures. The reason is its unique, patented fleece, which is inserted between the plastic film of the BIOSTAT CultiBag STR and the bag holding device of the tester. This fleece acts as a porous spacer, preventing direct contact between the bag and the smooth stainless steel surface of the holder. In addition, the fleece substantially reduces environmental heat transfer and, most importantly, eliminates the effects of masking of any potential pinholes. The high accuracy of the Sartocheck 4 plus Bag tester in measuring the pressure drop, along with its patented fleece technology, provides unrivaled reliability and reproducibility for testing.
The Sartocheck 4 plus Bag tester is an important contribution by Sartorius Stedim Biotech towards enhancing risk mitigation for single-use equipment in biopharmaceutical manufacturing.
Source:
http://www.sartorius.com/