The amazing speed with which therapeutic antibodies have come to the fore in the treatment of autoimmune disease, inflammatory conditions, and oncology, has pushed forward the race to develop novel monoclonal antibodies (mAbs)
The commercial value of these molecules is estimated at USD 85.4 billion in 2015 and should reach about USD 138.6 billion by the year 2024. New conditions are also being explored as potential targets for mAbs, including Alzheimer’s and Parkinson’s disease.
Antibody-drug conjugates, or ADCs, are also being developed to target specific cancer cells, being antibodies armed with radioisotopes or cytotoxic agents.
Clone Selection: The Bioprocessing Bottleneck of Antibody Manufacturing
The slowest step in developing a new mAb with therapeutic potential is clone selection. Many companies are racing to develop cell lines that can target specific cells based on physical, chemical or biological characteristics, and many of them are looking at similar or overlapping targets.
In addition, the cell lines produced must also be sturdy enough to withstand a manufacturing process. But clone selection today is hindered by legacy screening technologies. These include ELISA or Bio-Layer Interferometry (BLI), which can measure the immunoglobulin G (IgG)n titer, rather than indicating the health of the cell or the cell count itself.
This is a fallacy in that the cells that produce the most IgG may not be the healthiest ones which should be further developed. Another big obstacle with the use of these older techniques is the inability to analyze them because of the lack of integrated data analysis tools.
Legacy systems such as these mean repeated porting of data between several platforms, over days or weeks, which has an inbuilt possibility of introducing error.
Furthermore, the final output may be just a range of spreadsheets rather than a true depiction of the data collected.
Add to this the relative scarcity of vigorous candidate clones, and it is clear that despite the labor and investment of capital as well as of multiple resources, the process may take 6 to 12 months to succeed in discovering and optimizing a clone – and even then, the clone selected may not be the best, considering that only IgG levels were measured and not the overall cell attributes.
Breaching the Bottle Neck with a Disruptive Fast-Track Antibody Screening Platform
This problem of obsolete methods of clone selection has been met with the Sartorius Cy-Clone™ PLUS, an assay kit which has undergone validation for its use in selecting clones. It accelerates mAb production cell line generation.
The Cy-Clone PLUS assay in combination with the Sartorius iQue® Screener PLUS and ForeCyt® software, provides a powerful analytical tool that generates high content multi parametric data and helps determine whether to go ahead with a cell clone or not in a single-path workflow.
The iQue3 PLUS can assay as little as 10 µL, in a 384-well format plate, with zero dead volume. This microfluidics acquisition capability cuts cost by reducing reagent usage, thus enabling remaining sample to be retained for future use. The key value that it provides is the ability of simultaneously reporting on the following cell health and cell number data points:
- Secreted IgG
- Total cell number
- Total viable cell number
- IgG content per viable cell
- IgG per cell
- IgG per cell per day
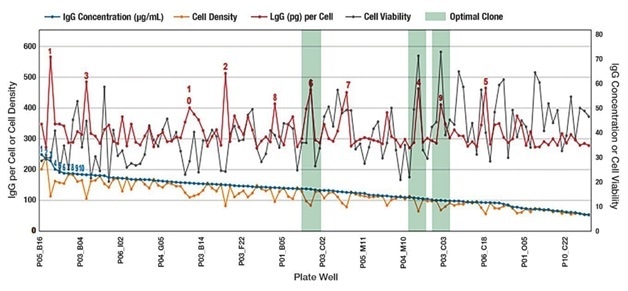
Figure 1. Example of simultaneously screening for IgG titer and cell health attributes. The blue line represents IgG concentration and clone ranking that would be typical of legacy technologies measuring only IgG titer such as ELISA or BLI. A more complete story —and the advantages of the Sartorius integrated platform capability—is told in the additional data points relating to cell number and cell health. The red line is IgG quantitation per cell. The gray line demonstrates that while the IgG concentration declined there are still several very viable clones. The green shading shows clones that have high IgG titers on a per cell basis. These clones could be interesting candidates for downstream processing and would likely have been excluded based solely on ranking by IgG titer. By combining separate time-consuming steps in cell line screening processes, the Sartorius platform saves time, while providing valuable information on clone productivity.
Single-Path Workflow: Increased productivity by combining time intensive screening steps
The prolonged upstream clone selection combined with the short period allowed under patent law for exclusive marketing rights, makes the time to production a crucial factor in determining whether one is successful in bringing a new therapy to the market.
This is where the integrated workflow offered by the Sartorius Cy-Clone PLUS assay streamlines this workflow and addresses the “wish list” of researchers:
- It enables the direct transfer of cells and supernatant without dilution steps, thereby achieving target concentration while saving time and minimizing transfer errors.
- No-wash assays eliminate tedious pipetting centrifuging and dispensing, thus conserving reagent in each step of the process.
- A wide dynamic range means that unknown samples need not be diluted, again saving much labor.
All this means that setting up a Cy-Clone PLUS assay takes only about an hour. Data acquisition is also simplified because of the included USB drive that contains a predefined template. The iQue3 PLUS begins to acquire data immediately, and is completed within 20 minutes for a 384 well plate.

Figure 2. Mix and Read Assay Workflow: Transfer undiluted cell suspension into the plate, add in the assay components and then read on the Sartorius iQue3 PLUS.
ForeCyt-Optimize, Analyze, Visualize, Realize...Faster!
The integrated ForeCyt Software is fast and efficient at generating data on IgG clones which guides the course of action in the clone selection process. The data may be in the form of cell-line plate heat maps, plots, dose response curves, histograms, and profile maps.
In addition, Panorama, a feature of the ForeCyt software allows multi-plate analysis to produce a large-scale picture that compares, identifies and ranks all the IgG clones across the entire experiment as shown in Figure 3.
Additionally, Panorama has criteria threshold slider bars that can adjust data to analyze for possible alterations and hypothetical changes in real time.
Panorama allows the researcher to request an optimized array of IgG clones for an experiment, thus eliminating the weeks and months of loading and reloading, along with multiple recalculations, that were associated with older tools.
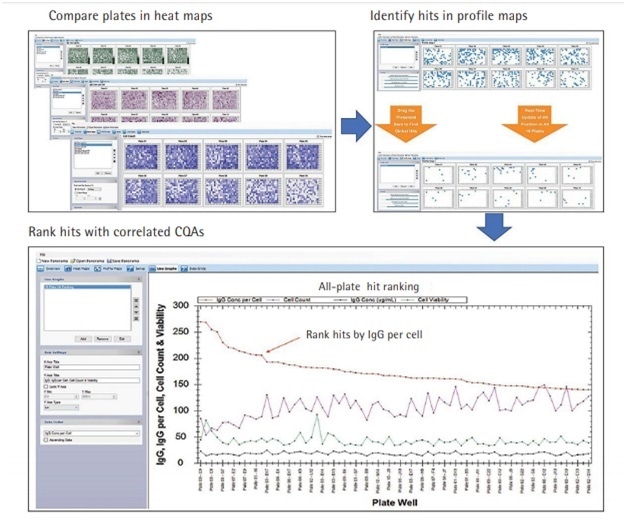
Figure 3. Cross-Plate Data Analysis Identifying Best Productive IgG Clones in a Screening Campaign.
Multiple Upstream Bioprocessing Advantages
The Cy-Clone PLUS forms a winning combination with the iQue3 PLUS and ForeCyt Software in the study of antibodies and other biomolecules. The iQue3 PLUS adapts equally well to manual processing, automated processing of fluids and robotic handling of assay plates. Other biological material processes that benefit from the use of Cy-Clone PLUS (Figure 4) include:
- Monitoring cell health and IgG
- Optimizing media, feed, and bioreactor characteristics
- Process monitoring
The Cy-Clone PLUS also boasts a multiplex reagent portfolio, accounting for the PLUS, which means it will in future be able to handle more CQAs, such as:
- Apoptosis
- Autophagy
- Proliferation
- Antibody binding
The development of mAb-based products is vitally important in dealing with diseases that place immense burdens on social and financial resources. Thus the use of this system will shorten the time from bench-to-bedside, impacting both patient care and profitability in a positive way. With mAb-based product development addressing diseases of such
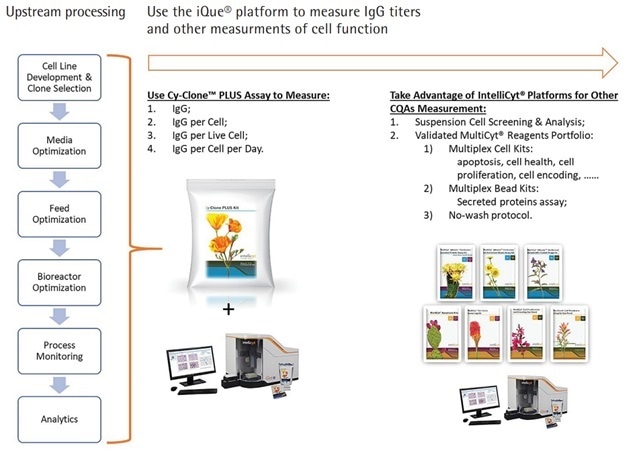
Figure 4. Use Cy-Clone PLUS and iQue3 PLUS Platform to Measure Multiple Readouts in Whole Upstream Processing of Human IgG Therapeutic Proteins.
References
- Monoclonal Antibodies (mAbs) Market Size Worth $138.6 Billion By 2024 https://www.grandviewresearch.com/ (accessed May 26, 2017).
- Novel monoclonal antibodies show promise for Alzheimer’s disease treatment https://www.sciencedaily.com/releases/2015/07/150720154218.htm (accessed May 26, 2017).
- Weiner, G. J. Nature Reviews Cancer 2015, 15 (6), 361–370.
- Wiley StatsRef: Statistics Reference Online 2014.
- Lai, T.; Yang, Y.; Ng, S. Pharmaceuticals 2013, 6 (5), 579–603.
 Sartorius
Sartorius
Sartorius is a leading international pharmaceutical and laboratory equipment supplier. With our innovative products and services, we are helping our customers across the entire globe to implement their complex and quality-critical biomanufacturing and laboratory processes reliably and economically.
The Group companies are united under the roof of Sartorius AG, which is listed on the Frankfurt Stock Exchange and holds the majority stake in Sartorius Stedim Biotech S.A. Quoted on the Paris Stock Exchange, this subgroup is comprised mainly of the Bioprocess Solutions Division.
Innovative Technologies Enable Medical Progress
A growing number of medications are biopharmaceuticals. These are produced using living cells in complex, lengthy and expensive procedures. The Bioprocess Solutions Division provides the essential products and technologies to accomplish this.
In fact, Sartorius has been pioneering and setting the standards for single-use products that are currently used throughout all biopharmaceutical manufacturing processes.
Making Lab Life Easier
Lab work is complex and demanding: Despite repetitive analytical routines, lab staff must perform each step in a highly concentrated and careful way for accurate results.
The Lab Products and Services Division helps lab personnel excel because its products, such as laboratory balances, pipettes and lab consumables, minimize human error, simplify workflows and reduce physical workloadsSponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.