The role of the pancreas is to control glucose levels in the blood. Normally, it does that through a combination of two hormones that it secretes: insulin and glucagon.
When glucose levels rise, i.e. when you've eaten something, insulin is secreted to promote the uptake of glucose into body cells. When insulin is not present, glucose levels rise to very high levels.
The B cells, otherwise known as the islet cells, secrete insulin in the pancreas. Essentially in type 1 diabetes those cells have been destroyed.
The accepted dogma now is that they have been destroyed by an autoimmune process, i.e. the body has, for some reason, recognized those cells as being foreign and has destroyed them.
Thus in a type 1 diabetic, no insulin or very little insulin is secreted.
How is type 1 diabetes currently treated and how did the idea to create an artificial pancreas originate?
We've now worked out that if we can give people insulin by injection, we can help control their blood glucose.
Type 1 diabetics do that by carefully counting the amount of carbohydrates that they eat. They're trained to count their carbohydrates so that they know how many they are going to eat in a meal and can inject the right amount of insulin before they eat so the blood glucose levels don't go too high.
To also help that, they test their blood. They test their blood glucose levels using blood glucose meters. They currently do that by pricking their finger and touching that blood to a test strip that sits in a handheld meter that 5 seconds later gives them their blood glucose level result.
Based on their blood glucose level results and the amount of carbohydrates they consume, they adjust the amount of insulin that they take. That's simplistically how it's done.
There are nuances around that. Some people take a basal level of insulin that is long acting. This keeps them at a steady state level and then they can just give little injections around meals to compensate for the amount of carbohydrates. Some people just do injections around meals.
That treatment has transitioned over the years to the development of, instead of injectable insulin by a syringe or a pen, and now insulin pumps. Insulin pumps are devices that inject the same type of insulin the patient uses in a syringe, but inject it in minute quantities over the whole day. Each cartridge lasts about three days, based on the stability of insulin and the amount of insulin you can store in a pump, and allows you to continuously infuse insulin.
The idea for creating an artificial pancreas came many years ago, even before the advent of today's continuous glucose monitor, which is the next step to enable the artificial pancreas to proceed.
According to the recommendations from the American Diabetes Association, most diabetics are recommended to test their blood by using a needle stick to prick their finger to measure blood glucose at least four times a day.
Type 1 diabetics and those who are brittle, i.e. those whose blood glucose levels vary widely, might test their levels more than 12 times a day.
To use a glucose monitor, a small sensor is inserted through the skin subcutaneously either on the abdomen or the back of the arm that essentially monitors the blood glucose and gives the patient the result every 5 minutes. It's controlled by a handheld device so at any time you can press the button and it will tell you what your glucose level is, and it will also tell you whether you're trending up or trending down.
When you do a finger stick, it's very much like taking a photograph: it's a snapshot of where your glucose is at the instant you test it. Unless you’ve done lots of glucose finger sticks, you have no idea whether your blood glucose is going up or going down from that snapshot. With a continuous glucose monitor, you get something else that is giving you results continuously every 5 minutes, so you can see what the trends of the results are, and what is happening. It gives you much more information and this usually helps with better control.
There is lots of published data from Juvenile Diabetes Research Foundation (JDRF) and from Joslin Clinic that shows continuous glucose monitors do promote better control of blood glucose in type 1 diabetics.
Now with the continuous insulin infusion, and the continuous glucose results you have two of the elements for an artificial pancreas. You know what the glucose is doing and you can control the amount of insulin that you are delivering, and therefore potentially you have the opportunity to close the loop, as it's called, and create that artificial pancreas.
The very first ideas of creating an artificial pancreas go back many years, however, the technology wasn't really there to enable it to happen.
It was only over the last 7 or 8 years as pump technology has improved, and continuous glucose monitors have appeared and have been approved by the FDA etc. that the elements have been there.
The third criteria for creating an artificial pancreas is the algorithm: the brain that basically takes the information from the continuous glucose monitor with the amount of insulin and the diet, (the number of carbohydrates being taken), and puts that all together and modifies the insulin delivery based on that information.
It's that algorithm that comes from the group at Addenbrooke’s Hospital in Cambridge, UK. There are also groups in San Diego, Padua, as well as lots of money being pumped in by Juvenile Diabetes Research Foundation that are now looking at different algorithms and how you take that information and basically feed it into the system to finally create that artificial pancreas.
This would allow a type 1 diabetic, to a certain degree, to be able to forget about his or her disease.
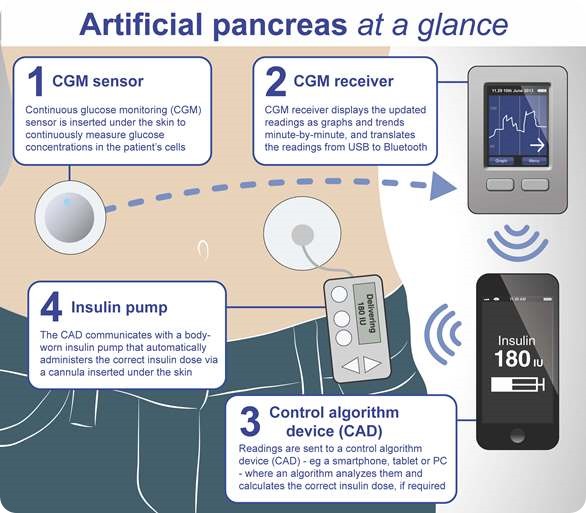
How would an artificial pancreas operate?
Essentially, what an artificial pancreas would be doing is taking the blood glucose results from a continuous monitor and taking the data that the patient puts in about the amount she/he is eating, and then adjusting the amount of insulin being delivered by the pump based on a mathematical formula that would be very personalized to that patient.
So essentially the device would mimic what the pancreas does.
What is Cambridge Consultants role in developing this device?
Dr Roman Hovorka at Addenbrooke’s has trialed this system in a clinical setting where they brought in type 1 diabetics and treated them during the day or overnight. In fact, I was involved with him before I joined Cambridge Consultants doing some work on behalf of Cambridge Enterprise, which is a technology transfer group of the university.
It started off with a nurse typing into a computer the blood glucose results and the carbohydrates. They then received the output of the algorithm and manually adjusted the pumps.
That transitioned into an ultra-portable PC by the side of the beds in some of the early in-home studies.
What we've been contracted to do is basically turn that into a mobile system such that the testing of the algorithm in real life, i.e. patients at home going about their daily business, is enabled. They can basically carry on with their life while this system can operate to control the insulin and as well as be monitored from afar i.e. by the group at Addenbrooke’s during the clinical trial.
So, what you're seeing is step wise progression of the testing from a clinical setting, to overnight at home, to numbers of days at home, to weeks and then months and so forth. Ultimately, we would hope from a development point of view that the system becomes approved.
But we are still working toward a final product yet. What we’ve done is used our wireless expertise to help two pieces of kit, a CGM and a pump, talk to each other by what we call a translator. It takes the signal from the CGM and transmits it into a phone or a tablet that’s got Roman’s algorithm on it, and that is the piece of equipment communicating to the pump. The pump then changes the amount of insulin being delivered (within certain parameters) to better control the blood glucose of the diabetic.
There is more work to be done on the productization of this in the future. Ultimately, we need to decide where does the ‘brain’ sit? One possibility is the brain could sit in a handheld phone. So essentially, the pump and the CGM communicate with your smart phone and the smart phone tells the pump wants to do.
You could also have a dedicated handheld device that controls both the CGM and the pump that qualifies as a medical device and you just carry the one device around and it controls both elements as it sits inside that device.
At what stage of development is the artificial pancreas currently at?
There were a number of presentations at the ADA American Diabetes Association meeting in Chicago recently all doing similar kinds of studies that Dr Hovorka at Addenbrooke’s has done, or is looking to do. These were early stage testing of systems employing the elements that we've just talked about: an algorithm, a CGM and a pump.
Right now every device appears to be at the early clinical trial validation point at the minute. I think there are some groups that are further into incorporating it into a potential product. However, we don’t know whether they have picked the right algorithm at this stage.
So early clinical testing, stepping through more and more real life settings is pretty much where trials are at the present moment.
How will this device differ from other systems such as the nurse-assisted system previously trialed in a hospital setting?
Essentially, everything is more self-contained, and there will be no interference from the nursing group at all.
If I was a type 1 diabetic, I'd have this device in my pockets, and the CGM and pump attached to my body and I would be carrying on my life as normal.
This system also provides the ability for the clinical trials group to remotely monitor what's going on via communication with the tablet. So the CGM data, carbohydrate data, and the instructions given to the pomp, would all be sent to a central server where the clinical trials group will be able to monitor both the safety and efficacy on to a day to day basis?
There are built in controls into all insulin pumps today, which mean that there is a maximum amount of insulin that can be delivered at any time. So the safety of these systems in clinical trials is assured because what the algorithm is doing is making fairly small changes to the currently programmed insulin delivery.
So they're not making gross changes of insulin delivery based on what people are doing, and as a result there’s a lot of safety built into this trial based on the inherent safety of insulin pumps.
How long do you think the development process will take and what hurdles must be overcome before this device can be made widely available?
I think it will depend on exactly how this is presented to the regulators, the FDA for example. I think it will also have higher hurdles to overcome if somebody is looking to use a smart phone as the controller.
We already know that the FDA is putting out guidelines and is very concerned about Medical Devices on smart phones, because the phone is not a medical device, and is not reviewed as such.
For example, if somebody brings this to the marketplace with a purpose built controller (it might look like a smart phone, but it isn't), that might be more acceptable to the FDA and minimize hurdles.
At the present there are both devices and pumps with purpose built handheld controllers. So, it’s not too different to say you're going to combine the communication and the control of both the pump and the data from your continuous glucose monitor into one device that just happens to have an algorithm in there that takes in data and makes automatic changes based on glucose and carbohydrate levels.
The ultimate driver of acceptability will be the quality of the data. The data must be is persuasive, of high quality, very thoroughly done and delivered.
The safety of the patients will be paramount. In my view, some safeguards are already built into pumps today, and I think that the arguments will be that we are not making gross changes here, we are just smoothing things and that smoothing gives much better control, better HBA1C's, etc.
Because of the way the data is monitored, those trials will likely be pretty extensive and subsequently I think we’ll see the device available within the next five years.
What impact do you think this artificial pancreas will have on the lives of type 1 diabetes patients?
It will allow them, to a certain degree, to forget their disease.
Patients who are a type 1 are usually diagnosed as children so they may have been living with diabetes for many years. To get to that stage where you can actually forget about it, I suspect will be a tremendously liberating feeling. Of course this will only be once they gain confidence in it.
There is a type 1 diabetic named Kelly Close who publishes a blog called Close Concerns. She has had the opportunity to wear one of these devices. Her response was, “suddenly I can forget about my disease.”
The upside for the diabetic is better control of blood glucose. This means fewer complications, which means less hospital visits. Less hospital visits means less costs to the Healthcare System. So everybody wins.
What are Cambridge Consultants plans for the future?
We have skillsets that we hope will be recognized by people developing products in this area that would help them bring these kinds of products to market place, in terms of our communication, wireless skills and product development skills.
We are considered to be one of the best technology consultants in terms of product development in the world and we would hope to be involved in other developments in this area, whether it be in a digital health way - in terms of how is that data that managed, and how is that data transmitted and then sent to doctors and sent to patients afterwards - or purely the productization and commercialization of ideas.
Where can readers find more information?
About John Pritchard
 John Pritchard joined Cambridge Consultants in 2012 and is the Commercial Director for Diagnostics and Life Sciences in Cambridge Consultants Ltd’s Global Medical Technology Practice. In this role he is responsible for business development and commercial activities in this market segment. John brings over 30 years of experience in diagnostics from companies on two continents and he has held senior roles in R&D and management in both large corporations and University spinouts. He spent 4 years on the UK Medical Research Council’s DPFS panel. John received his PhD is from Liverpool University, a joint programme between Biochemistry and Surgery, and his MBA from Boston University.
John Pritchard joined Cambridge Consultants in 2012 and is the Commercial Director for Diagnostics and Life Sciences in Cambridge Consultants Ltd’s Global Medical Technology Practice. In this role he is responsible for business development and commercial activities in this market segment. John brings over 30 years of experience in diagnostics from companies on two continents and he has held senior roles in R&D and management in both large corporations and University spinouts. He spent 4 years on the UK Medical Research Council’s DPFS panel. John received his PhD is from Liverpool University, a joint programme between Biochemistry and Surgery, and his MBA from Boston University.