New biosensor-based technology could help to reduce the over-prescribing of antibiotics by giving GPs and other frontline healthcare staff an accurate and fast method of identifying the nature of infectious diseases.
The innovative hand held device developed by the UK-based OJ Bio Ltd. uses specially developed biochips with a dedicated reader and supporting software carried on a mobile phone app or a PC.

When biological samples, from serum, saliva or blood, are applied to the biochip (held in a disposable cartridge), the presence of a disease antigen is translated into an electronic signal which is then converted to display the result of the test on the supporting software.
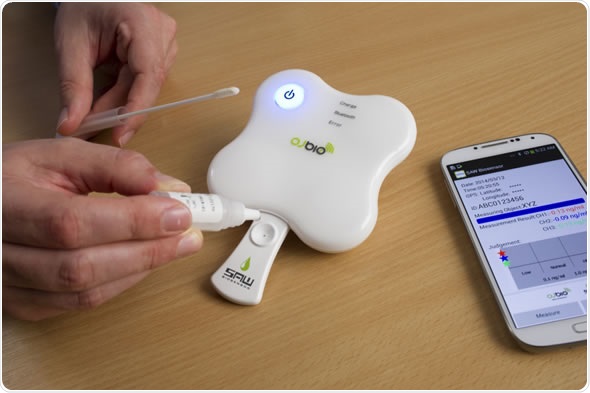
Formal clinical trials using the device are due to start in 2015 for the detection and diagnosis of the influenza A and B viruses, common flu strains previously linked to some major epidemics, and Respiratory Syncytial Virus (RSV), a major cause of coughs and chest infections.
In addition, the device can also be used to detect the presence of C-reactive protein - a biomarker of inflammatory disease, which can be used to rule out serious bacterial infections and which has therefore been proposed as a useful tool in the reduction of the inappropriate prescribing of antibiotics.
Dale Athey, chief executive of OJ Bio, said: “Our new device provides a low cost test that dramatically improves the speed of diagnosis and treatment of diseases without sending samples to the laboratory.
“Currently, GPs and frontline health workers have no easy way of determining if a patient with flu-like symptoms is suffering from a bacterial or viral infection and may therefore take the cautious decision to prescribe antibiotics. However, by providing an immediate indication of a lack of bacterial infection at the point of care, the device removes this uncertainty, helping the patient to understand why antibiotics may not be necessary and are not being prescribed.”
The state of the art biosensors at the heart of the OJ Bio system are a unique fusion of advanced technology from its parent companies, UK nano biotechnology specialists Orla Protein Technologies Ltd and leading Japanese electronics company Japan Radio Company Co, Ltd.
The surface acoustic wave (SAW) electronic chips developed by JRC are coated with disease-specific biocapture surfaces developed by Orla. The presence of a disease antigen causes a shift in the phase angle of the surface acoustic wave passing across the chip surface and this is translated into an electronic signal.
This signal can then be detected and its presence (or absence) determined, providing an unequivocal pass or fail result for the particular disease being tested for.
Bluetooth connection of the reading device to special diagnostic software enables the test results to be displayed within seconds. Unlike other rapid test systems this can also provide a measure of the target biomarker, rather than just a simple yes or no result.
The platform technology allows the detection of protein biomarkers, meaning the application areas are very broad.
Bio assay development is continuing in the UK and manufacturing facilities are already under development in Japan. There are opportunities for technology access and licensing.
Source: www.oj-bio.com