May 12 2015
New “Map” of Protein Helps Researchers Understand Cellular Transport
Scientists from The Scripps Research Institute (TSRI), working closely with researchers at the National Institutes of Health (NIH), have mapped out the structure of an important protein involved in cellular function and nervous system development.
The new structure provides crucial information for understanding how the protein binds to cellular components. It's also the first structure determined of any ligase in the tubulin tyrosine ligase-like (TTLL) family.
Scientists have been especially curious about the role of TTLLs because mutations in these proteins have been linked to a range of neurodegenerative diseases, including retinal dystrophy and the rare Joubert syndrome.
"This protein is highly expressed in the nervous system and has an integral role in neuronal development," said Elizabeth Wilson-Kubalek, senior staff scientist in Professor Ron Milligan's laboratory at TSRI and co-first author of the new paper with Christopher Garnham and Annapurna Vemu of the NIH's National Institute of Neurological Disorders and Stroke (NINDS).
The new research was published online ahead of print by the journal Cell.
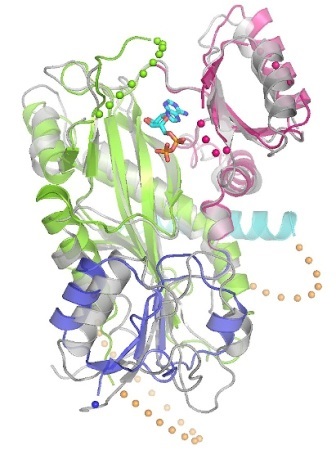
The new image of TTLL7, from a family of proteins that has been linked to neurodegenerative diseases, provides critical information about how the protein works.
Cellular Highways
While previous research had shown that TTLL7 modifies microtubules (hollow tubes that transport cellular components and act as highways and scaffolding in the cell) by adding one or more molecules of the amino acid glutamate, exactly how has remained a mystery. The new study goes a long way to answering that question.
In the new study, the researchers saw for the first time how three positively charged regions of TTLL7 interact with the microtubule substrate. Most importantly, they found that the active site of TTLL7 is ideally positioned to contact the negatively charged "beta-tail" of beta-tubulin, one of the two protein building blocks of the microtubule polymer (alpha- and beta-tubulin). The alpha and beta "tails" that protrude from the microtubule surface are known sites for modification, which in turn, determine which motors and associated protein will bind to the microtubule.
These findings add to the growing understanding of the "tubulin code"—a phenomenon where TTLL7 and similar proteins add amino acids to microtubules and prompt them to fast-track certain proteins for transport.
"It's like opening up new lanes of traffic," said Gabriel Lander, an assistant professor at TSRI and a co-author of the new study.
A Piece of a Bigger Puzzle
The researchers were able to solve the structure of TTLL7 by combining x-ray crystallography and electron microscopy (EM). The team at NIH, led by the paper's senior author Antonina Roll-Mecak, used x-ray beams to create an atomic structure of the crystallized TTLL7 protein. The team at TSRI then used electron microscopy, which pelts samples with high-energy electrons, to see what TTLL7 looks like when bound to a microtubule. The atomic structure from the NIH team could be fit into the lower-resolution 3D EM image reconstruction to identify the regions of the protein that interacted with the microtubule surface.
"Merging two structural techniques and biochemistry led us to a more complete story," said Wilson-Kubalek. "It's really exciting to see something no one has ever seen before."
Wilson-Kubalek compares the research to putting together a puzzle. With this new piece in place, the researchers can tackle new aspects of microtubule function and work toward applications in human health.
"The take-home message is that this is the first time we've seen how this protein sits on the microtubule, and this is going to be of major importance down the road," Wilson-Kubalek added.
Source: http://www.scripps.edu/