Jan 11 2016
The global supply of oral cholera vaccines is set to double after WHO approved a third producer, helping to address global shortages and expand access in more countries.
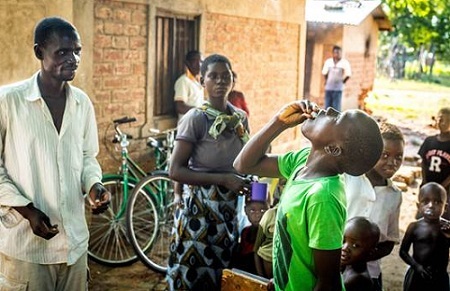
Oral Cholera Vaccination Campaign in Southern Malawi, 2015. WHO/L. Pezzoli
Globally, OCV production is low, with demands currently exceeding supply. Sudan and Haiti last year made requests to WHO for supplies of vaccines to conduct pre-emptive vaccination campaigns that could not be filled because of the global shortage.
The vaccine producer, a South Korean company, is the latest oral cholera vaccine (OCV) manufacturer to be approved under the WHO’s pre-qualification programme, which ensures that drugs and vaccines bought by countries and international procurement agencies such as the United Nations Children’s Fund (UNICEF) meet acceptable standards of quality, safety and efficacy.
The addition of an additional pre-qualified vaccine producer is expected to double global supply to 6 million doses for 2016, with the potential for further increased production in the future. This additional capacity will contribute to reversing a vicious cycle of low demand, low production, high price and inequitable distribution, to a virtuous cycle of increased demand, increased production, reduced price and greater equity of access.
Cholera is an acute diarrhoeal disease that can kill within hours if left untreated. There are between 1.4 million and 4.3 million cases a year, and as many as 142 000 deaths. Cholera is endemic in more than 50 countries, but usually only garners international attention during humanitarian emergencies, such as the outbreak among refugees in Goma, Democratic Republic of the Congo, in 1994 that killed tens of thousands. Climate change and El Niño may also be contributing to more frequent cholera outbreaks.
Oral cholera vaccines have been used in mass vaccination campaigns in response to humanitarian emergencies since 1997. But because the disease disproportionately affects poor communities who are often unaware that the vaccines exist, there has historically been little demand for the products.
In 2013 the WHO created the world’s first OCV stockpile, undertaking to buy and use 2 million doses a year in order to stabilize and create demand for the vaccines.
Vaccination requires 2 doses per person, meaning the stockpile is sufficient to cover 1 million people.
Access to OCV has been further improved by a commitment of US$115 million over 5 years from Gavi, the Vaccine Alliance, to expand availability and the use of vaccine in countries with endemic cholera. Since the OCV stockpile was created more vaccines have been distributed and used than in the previous 15 years. A total of 21 OCV deployments of about 4 million doses to 11 countries have been used in various contexts: humanitarian crises in Cameroon, Haiti, Iraq, Nepal, South Sudan, and Tanzania; outbreaks in Guinea and Malawi; and in endemic hotspots such as Bangladesh and the DRC.
The creation of the stockpile and pre-qualification of a new vaccine producer highlights the success of an international joint effort through public-private partnership, including governments, non-profit organizations, manufacturers, donors and research organizations. Accompanying the use of stockpile vaccine, many donors and partners have worked together within the framework of the Global Task Force on Cholera Control (GTFCC) to demonstrate the public health potential of this vaccine when used in mass vaccination campaigns. The evidence is contributing to a body of work which will inform larger investments on the further production and use of this vaccine.