Mar 2 2016
Researchers show that the protein CCN4 positively regulates the generation of cartilage matrix, which are depleted in osteoarthritis.
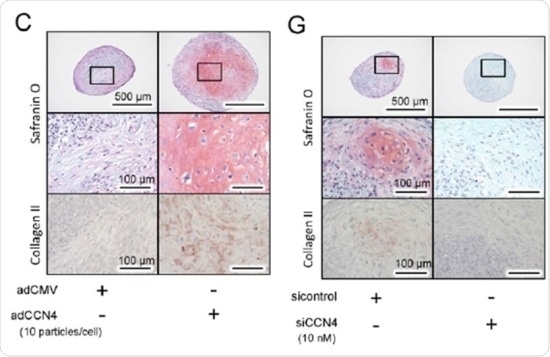
The effect of CCN4 on the chondrogenic differentiation of hBMSCs in vitro. Twenty eight days after chondrogenic induction, histological analysis of hBMSC micromass cultures was performed for detection of glycosaminoglycans with safranin O staining, or type II collagen alpha 1 by immunohistochemistry. Micromass cultures with adCCN4-transduced (CCN4 overexpressed, left) shows intense immunostaining for collagen type II. In contrast chondrocyte-like cells were nearly absent, and ACAN and COL2A1 transcript levels were also significantly down-regulated in siCCN4-transduced (CCN4 knock down, right) hBMSCs micromasses.
Osteoarthritis is the most common musculoskeleton disease. It is caused by the loss of articular cartilage and subchondral tissue, which causes pain, stiffness and a loss of mobility in joints. Adult cartilage does not readily regenerate, and the disease is often treated by implantation of patient-derived ‘chondrocyte cells’ – the cells that make up cartilage. As a result, the mechanisms behind the differentiation of stem cells into chondrocytes have attracted a great deal of interest. Researchers at Okayama University Graduate School of Medicine and colleagues now report:
For the first time, we showed that CCN4 exerts a positive effect on [transforming growth factor beta 3] TGF-β3-induced chondrogenesis by modulating TGF-β3 binding to the surface of [human bone marrow stromal cells ] hBMSCs and enhancing its downstream signalling.
The CCN family includes 6 proteins some of which have known links to the generation of bone and cartilage but so far the role of CCN4 in chondrogenesis has been unclear. In the current study, Yuya Yoshioka, Mitsuaki Ono and colleagues at Okayama University Graduate School of Medicine, Japan, and the National Institutes of Health in Maryland, US, investigated hBMSCs that either over- or under-expressed the CCN4. By analysing micromass cultures of the hBMSCs, they found that cells overexpressing CCN4, led to enhanced TGF-β3-induced processes that produce cartilage. Where CCN4 was knocked out these processes were inhibited.
They also investigated cartilage repair in both wild-type and Ccn4 knock out mice after a surgical knee injury. Cartilage repair was significantly greater in the wild type mouse. The results make progress in understanding the cellular mechanisms in cartilage repair that may contribute to new treatments and improved clinical outcomes.
Background
Cartilage in the musculoskeleton
The cells in healthy articular cartilage are all the same type – chondrocytes, which are formed from by the differentiation of mesenchyme cells in the process of chondrogenesis. Chondrocytes synthesize the components of the cartilage extracellular matrix.
Fetal skeletons are largely cartilaginous until ossification replaces them with bone. However the joints remain cartilaginous. Cartilage is not readily repaired in adults, and injury or trauma, overloading through obesity or simply aging can all result in degeneration and depletion of articular cartilage and subchondral bone leading to osteoarthritis.
CCN proteins
The CCN4 protein belongs to a family of CCN proteins that have a unique modular structure of four distinct functional domains, which allows them to interact with a range of other proteins, cell membrane receptors and extracellular matrix components. They are known to regulate several cell functions, including adhesion, migration, proliferation, and differentiation both in vitro and in vivo.
Previous work has already demonstrated the roles of CCN2 in chondrogenesis through the proteins BMP-2, FGF-2, and IGF, as well as CCN4 in osteoblast differentiation through BMP-2. High expression of CCN4 is also reported for osteoarthritic mice models and human patients. To investigate the effect of the CCN4 in the current study, hBMSCs were transfected with adenovirus that expresses CCN4 to overexpress the gene and hBMSCs cultured in a medium with RNAiMax and SiRNA to knock down CCN4 expression.
The researchers note that CCN4 alone in vitro does not have osteo-chondrogenesis functions, suggesting that other proteins may intermediate CCN4 interactions with growth factors. Further work is needed to clarify these mechanisms.
Source: http://www.okayama-u.ac.jp/