Mar 16 2016
Formation of amyloid fibrils is a characteristic feature of neurogenerative diseases like Alzheimer's. As published in the journal Angewandte Chemie, German and American scientists have found evidence that these fibrils adopt several distinct three-dimensional architectures in real patient and animal tissues.
 If a protein misfolds, it is usually readily degraded into its harmless components by special proteolytic enzymes in the cell. However, under certain pathologic conditions, some proteins or polypeptide chains can form aggregates that stack together in very stable fibrils, the most prominent forms being the amyloid fibrils found in the brain tissue of Alzheimer' s patients. These fibrils can have different morphologies varying lengths and widths and in the structure of the crossover region where the polypeptides entangle. However, that was found for laboratory-grown samples, but do these morphologies also occur in real tissue? A team of scientists at research institutes in Germany and the United States led by Marcus Fändrich at Ulm University have thoroughly investigated extracts from animals and patients suffering from different forms of amyloidosis and found that yes, they do. Their result has important applications for possible treatment scenarios.
If a protein misfolds, it is usually readily degraded into its harmless components by special proteolytic enzymes in the cell. However, under certain pathologic conditions, some proteins or polypeptide chains can form aggregates that stack together in very stable fibrils, the most prominent forms being the amyloid fibrils found in the brain tissue of Alzheimer' s patients. These fibrils can have different morphologies varying lengths and widths and in the structure of the crossover region where the polypeptides entangle. However, that was found for laboratory-grown samples, but do these morphologies also occur in real tissue? A team of scientists at research institutes in Germany and the United States led by Marcus Fändrich at Ulm University have thoroughly investigated extracts from animals and patients suffering from different forms of amyloidosis and found that yes, they do. Their result has important applications for possible treatment scenarios.
In general, amyloid fibrils can be detected by dye binding, and special spectroscopic techniques give insight into the folding pattern of the proteins. However, this may not be enough for distinguishing these morphologies. "Such intra-sample polymorphism can be invisible to spectroscopic techniques and requires single-particle techniques," the authors say. Therefore, Fändrich and his colleagues employ electron microscopy techniques to identify the morphologies.
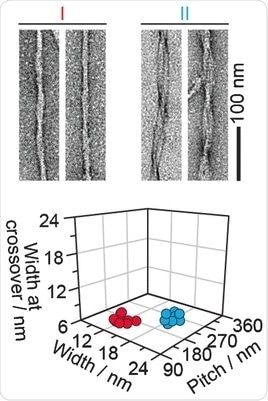
They extracted and investigated amyloid fibril structures from real diseased tissues. The first examples were taken from the hearts of two individuals suffering from so-called light-chain amyloidosis. The fibrils displayed at least two morphologies, the scientists found.
"Morphology I is thinner, while morphology II presents a more clearly resolved crossover structure," they write. The same was true for another sample from a patient who suffered from a transthyretin-amyloidosis, and also for animal samples like goat and mouse. All extracted fibril samples contained the fibrils in various and distinguishable shapes. Thus the scientists conclude: "The amyloid fibrils within a diseased individual can vary considerably in their three-dimensional architecture."
This result of pathogenic polymorphism within one individual is very important because it has impact on possible personalized medicine approaches for those forms of amyloidosis where the fibrils prove fatal: As patients develop more than one fibril structure, treatment of only a single fibril form would not be effective.