There are currently no medications which address the core symptoms of ASD, and a significant barrier to their development is that researchers have historically lacked effective methods for measuring clinical outcomes.
With ASD affecting 1 in 68 children in the U.S., the study and development of targeted therapies that effectively improve clinical outcomes and quality of life is becoming even more urgent, which is one of the reasons Janssen undertook Project JAKE™.
Please can you give an overview of the Janssen Autism Knowledge Engine (JAKETM)?
The Janssen Autism Knowledge Engine, or JAKE, is a first-of-its-kind, digital, integrated system of tools and technologies for facilitating data collection and advanced analytics in clinical trials.
JAKE’s software is designed to enhance and organize the type and quality of information collected about individuals with ASD with the hope of gaining insights into effective clinical measurements or markers, and ultimately contributing to the development of new medicines for patients and providing valuable information to the broader research community.
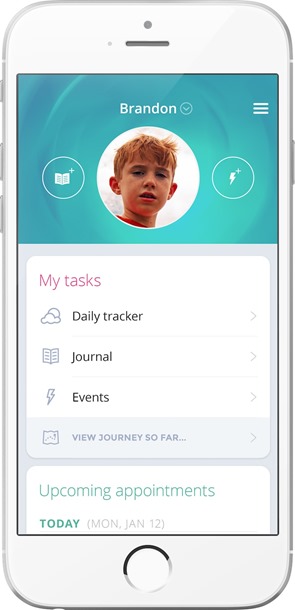
JAKE is comprised of three components – its web and mobile portal, biosensor platform and data pipeline – which are supported on most modern web browsers and mobile devices and allow researchers to capture and organize ASD-specific data:
- The JAKE Portal offers web and mobile tools for caregivers and researchers, including customizable calendar-like therapy trackers, clinical assessments, a tracking system that allows for logging of key events and information as it happens, free-text journaling that allows a parent or caregiver to write about daily events related to their child's autism and exchange messages with other members of the child's care team, as well as personalized reports and charting. The combination of these tools results in real-time trial tracking and adherence monitoring capabilities for researchers.
- The JAKE Research Biosensor Platform provides patients with sensors that can quantify behavior and offer new physiologic insights, allowing for the implementation of sensitive and objective endpoints. Some biosensors utilized by JAKE are designed to be worn daily, collecting data passively on a continuous basis, while others are designed to gather feedback during a discrete battery of “challenge tasks” administered in a lab setting.
- The JAKE Data Pipeline is driven by an analytics engine that is designed to construct a unique picture for each child and identify patterns in subgroups of individuals with ASD, resulting in dynamic profiles for respondents and ultimately meaningful data collection and output in clinical trials.
How was JAKETM developed?
The project was conceived through an internal program known as the Janssen Incubator, which supports the creation of “venture teams” focused on identifying highly innovative ideas arising from our scientific community in areas of potentially disruptive, cutting-edge research, which may lead to novel platforms, products or technologies.
The JAKE Venture brings together talent and expertise from key clinical, scientific, and information technology organizations to develop a robust approach to therapeutic assessment and development.
The team took an integrated collaborative approach to JAKE’s development, consulting with patient advocacy organizations and parents of individuals with ASD to provide scientific guidance, and leveraging the experience, capabilities and leadership of the Janssen R&D Neuroscience Therapeutic Area, as well as other scientific experts in the field of ASD and biosensor technology.
In what ways do you think JAKETM will impact autism research?
Ultimately we are trying to contribute to development of new medicines for patients and provide information to the broader research community. We’re starting our research with JAKE now, but we believe it may prove helpful in collecting data and producing better analytics in clinical trials.

How will JAKE’s technology affect the experience for patients and caregivers participating in clinical research?
Parents and caregivers of children with ASD deal with challenges on a daily basis, and we believe participation in a study should not add burden to their lives. JAKE aims to improve the experience of patients and caregivers participating in clinical research through the availability of user-friendly, online administration to complete scales and to-do lists, which can help parents with adherence to protocols. We believe that this will ultimately enhance the quality, including the ecological validity, of the data collected.
What further changes are necessary to overcome barriers in autism research?
As mentioned previously, one of the biggest barriers to advancing autism research has been that methods for measuring clinical outcomes, particularly for core symptoms, for subpopulation stratification and measuring change are not yet established.
Clinical care can be uncoordinated, lacking a clear way to easily organize and access data on diagnosis, comorbidities and treatments across the multiple providers. Measures of ASD symptoms, mainly paper and pencil or observational measures, are typically provided by clinicians or caregivers, and have historically been used for measuring symptom severity or to facilitate diagnosis – not to measure short-term change.
Furthermore, the ASD population is very heterogeneous, which complicates informed selection of clinical trial participants to participate in early proof-of-concept studies.
Our hope is that through developing the JAKE system for use in clinical development of new medicines for core ASD symptoms, we will be better equipped to address some of the challenges we face in the clinical ecosystem and ultimately help improve the lives of individuals with ASD.
There is also encouraging progress being made in the development of biomarkers for ASD through initiatives like the EU-AIMS Longitudinal European Autism Project (LEAP), a novel multi-center study being undertaken to help identify and validate genetic biomarkers for the treatment of ASD, and the Autism Biomarkers Consortium – Clinical Trial being conducted in the United States.
Where can readers find more information?
For those interested in finding out about our clinical research with JAKE, we recommend that you review open clinical trials on www.clinicaltrials.gov.
About Gahan Pandina, PhD
Senior Director, venture leader, Janssen research & development, Titusville, New Jersey
Gahan J. Pandina, PhD, is a Senior Director, Venture Leader in the Janssen Research and Development, Pharmaceutical Companies of Johnson & Johnson, in Titusville, New Jersey. His current role is as the venture leader of a team whose missing is to develop a system of tools and technologies to optimize novel medication development for Autism Spectrum Disorder (ASD). Over the past 14 years at Janssen, Dr. Pandina has served as the clinical leader for several global, Phase 2a/2b drug development programs in the areas of schizophrenia & cognition, ADHD, mood, and sleep disorders. He has worked on three successful Phase 3 registration programs in schizophrenia and ASD, and conducted a number of Phase 3B/4 studies in schizophrenia, mood, ADHD, and anxiety disorders. He is an Adjunct Clinical Associate Professor of Psychiatry at Rutgers-Robert Wood Johnson Medical School in Piscataway, New Jersey, where he teaches in the psychiatry residency and psychology internship programs. He is also a Visiting Professor in the Center of Alcohol Studies at Rutgers University in New Brunswick, New Jersey.
Dr. Pandina is a licensed clinical psychologist, and a member of several international societies including the American Psychological Association, Association of Clinical Research Professionals, and the International Society for CNS Clinical Trials Methodology. He is the author of numerous publications in both pediatric and adult psychiatry, psychology, and neuroimaging research in psychiatric disorders. He has a particular interest in clinical research on the efficacy and outcomes of psychiatric and psychological treatments. He serves on the board of two non-profit organizations focused on mental health needs.
Dr. Pandina received a Bachelor of Arts degree in psychology from the University of Vermont, and a PhD in Clinical Psychology from Binghamton University in Binghamton, New York, and completed doctoral fellowships in both Neuropsychology and Child and Adolescent Neuropsychology at the Robert Wood Johnson Medical School in Piscataway, New Jersey.