Please can you give an introduction to your research?
I’m a biophysicist at the University of Bordeaux and I mainly work on bio-imaging methods in the cancer laboratory research facility, mostly for brain cancers.
My group develops spectroscopy-based 3D imaging methods that are aiming to provide insight into the way tumors develop inside the brain.
3D Infrared Microscopy for Preclinical Pathology from AZoNetwork on Vimeo.
What is the significance of spectral imaging in biomedical studies?
Spectroscopy has been used in biomedical studies for quite a long time now, at least two decades. It involves the chemical imaging of bio-samples.
-Eds-150.jpg) Left: A mouse brain tissue section analysis by IR microscopy showing the anatomical details of brain with tumor (left part) based on extracellular matrix distribution. FTIR acquisition lasted 12h for a 20-µm resolution while QCL-IR acquisition could be done in 1h50 with a 4.4-µm resolution.
Left: A mouse brain tissue section analysis by IR microscopy showing the anatomical details of brain with tumor (left part) based on extracellular matrix distribution. FTIR acquisition lasted 12h for a 20-µm resolution while QCL-IR acquisition could be done in 1h50 with a 4.4-µm resolution.
The image shown is down-sampled by 8x8 due to file size limitations.
Spectroscopy and microscopy provide a global chemical picture of your sample and you can access different chemical parameters. A big advantage is that you don't need to label or stain your sample first.
We now have access to imaging methods using microscopy. Since the new microscopes are much faster, we have access to larger samples. You can even foresee the 3D reconstruction of the chemical information from the sample and turn that into biological information.
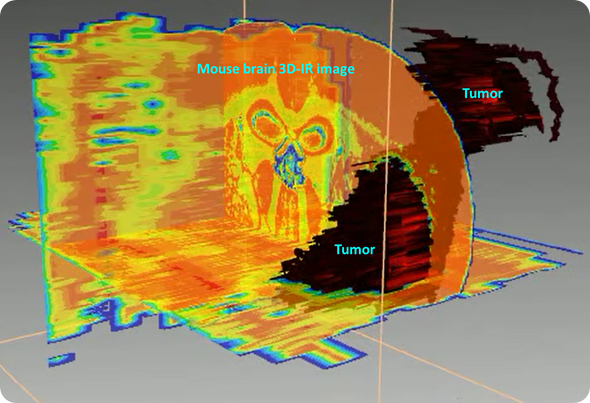
3D reconstruction of the tumor based on lipid/protein ratio. The tumor is presenting higher amount of proteins while brain is a “fatty” organ, thus making this ratio significantly different between tumor and healthy brain tissues.
This is changing the role of infrared microscopy in the panel of bioanalytical methods, because we can reconstruct information in three dimensions in a quantitative way and have access at the same time to metabolic, biochemical, structural and anatomical parameters that we could not consider before.
What are the main advantages of the Spero microscope from Daylight Solutions and how does it compare to other microscopes?
The first generation of microscopes was limited in some capacity of measurement because of the detectors they used, which were nitrogen-cooled. That meant a 4- or 5-hour capacity of measurement. That measurement time, combined with the fact that the source they used to provide the photon flux on the sample was quite weak, meant we had to accumulate a lot of scans.
Accumulating all those scans would mean we could not perform microscopy of very large sample areas. The weak source also meant the signal-to-noise ratio could not be maintained for very long. For example, in the case of a complete mouse brain, which represents 400 to 500 consecutive sections 10-µm thick, it would take almost a year to get everything done. No one is doing that because you cannot maintain the same quality of measurement between the first and last day.
The Spero microscope has two or three big advantages. The first one is that it uses a laser source, which is much more powerful than the previous sources found on commercial instruments, the Globar sources. It provides access to a very high signal-to-noise ratio, which can be maintained over a very long time for acquisitions.
That is coupled with a second advantage, which is that we now use detectors that do not have to be liquid nitrogen-cooled, and they are also larger. Together, these advantages mean you can use it for very long acquisitions and because it's 50 times faster than before, you can have access to a large data set in a much shorter time.
I would say that if you still consider the 400 to 500 2D mouse brain sections, now, you can get this information in just a few weeks and I think that very soon, it will be possible within just a few days. In that case, you have access to a very large data set, which can be reconstructed in 3D and then you can access information that you could not even imagine before.
If you have a quick turnaround in a matter of days or weeks, do you have the opportunity to affect the course of drug development?
Yes, for two reasons. The first one is that I think we cannot ask a big pharmaceutical company to wait a whole year for a result. Even if the drug development is taking years, they cannot wait another year for a result. If it instead took weeks for us to provide a result, that would be in the time frame of their work, which is fine.
The other point is that we have access to information from the sample which is supervised or non-supervised. Usually, in histology, you are searching for something predetermined and you use labels for such specific parameters. With IR microscopy, you still have access to that, but you can now also have access to many different kinds of information.
For example, you can take a normal sample and a pathological sample that is treated with a drug and check everything that is different between them. It’s all based on the chemical contents of the sample, whatever their origin, and IR spectroscopy provides a global view of these contents. Then, you can check every effect the drug is having on the organ or tissue.
This is what spectroscopy can do and what labeling cannot do, because spectroscopy is non-supervised. You have access to a very large range of chemical information and this is the richness of spectroscopy. You can check parameters off. You can check a parameter that you would not expect to move because of a treatment and then find that it does move and that's making the breakthrough with conventional histology.
Can you give an example of the projects that you've been able to undertake using the Spero?
In this lab, the main activity is histology. This has already been carried out by different groups across the world for a couple of decades. It was limited to small dimensions, let's say a few square millimeters only.
Now, we have access to larger dimensions, in 2D and in 3D. In 2D, it can be, for example, a complete mouse brain section or a complete biopsy section, if we are talking about biological and clinical applications, respectively. We can do that for a stack of successive sections and with that, we can create a 3D reconstruction. This is important, because it is changing the face of infrared microscopy.
Since we have access to 3D information, we can resolve anatomical, biochemical and structural parameters that were not visible with classical histology. Basically, what we are doing is 3D pathology, which is how much we can show that disease is organized in 3D in the biopsy, in the animal organ, in different samples.
The first basic application is clinical; we can use it for diagnostics. We can analyze a biopsy to section it and reconstruct the 3D information in that biopsy. The second kind of application is pharmaceutical; we can check all the effects of drugs on animal organs. This is the pre-clinical validation of drug effects on the different organs. And because 3D IR microscopy provides a global chemical analysis of the organ, the preclinical analysis of drug effects is becoming systematic. That could not be envisaged before.
3D Histology with the Spero™ Infrared Microscope
3D Histology with the Spero™ Infrared Microscope from AZoNetwork on Vimeo.
What problems has the Spero enabled you to overcome and what challenges do you think are on the horizon?
I think that the Spero microscope has removed one main difficulty we had before, which was not having access to large acquisitions while maintaining a common quantitative scale between all spectra. This is the first point, which is mandatory, because this is opening the way to 3D applications.
3D applications will now raise the main problem, which is the big data issue, both in terms of complexity and size. If I take the mouse brain example, one single brain will be several terabytes of spectral data. That several terabytes will be turned into other terabytes of data, called metadata, which are biological parameters we can reconstruct from the chemical images.
We can resolve the blood system; we can resolve the distribution of cells; we can resolve different structural parameters which are parameters of the tissue, in 3D. Now, we have to compute everything for the spectral data treatments and also for the 3D visualization of the parameters. The 3D visualization will be a big challenge for 3D microscopy now, using infrared microscopy.
What is the role of computing and mathematics in these studies involving 3D-IR image processing?
The main problem with computing is that, again, we have terabytes for every 3D image. This means that infrared microscopy is now turning into something completely different. We cannot use standard computers to make some basic data treatments; we need a computer system that is able to handle both mathematical treatment and visualization; we need to merge those.
We are defining a computer which is able to merge CPU and GPU models of mathematical data treatments. These mathematical data treatments are first based on how much spectral data we can extract from the 3D spectral matrix of an image and how we can turn that into mathematical information that we can manipulate to reconstruct all the information.
We need to define a software suite which is able to analyze all the information, which is quantitative at the beginning and has to remain quantitative to the end. For infrared microscopy and for chemical imaging in general, this is quite new and nobody is doing it.
When you carry out imaging, you usually have a single parameter; you have an intensity of a single parameter of your image. Now, we have an image, which, for every pixel, is a few hundreds of parameters. The last dimension of the voxel is this one.
From this, let's say 200 to 300 wavelengths we have per spectra, we extract a couple of hundred infrared bands. From these 200 hundred infrared bands, we extract biological information or we construct biological information. This means the mathematics is becoming a really big part of the data treatment now. And we are not just compiling existing algorithms to do it, we have to create most of them from scratch, designed specifically for 3D spectroscopy.
3D Infrared Microscopy - A Multidisciplinary Technology Chain
3D Infrared Microscopy - A Multidisciplinary Technology Chain from AZoNetwork on Vimeo.
Can you give an overview of the range of applications of 3D-IR microscopy?
I would say that at the moment, 3D infrared microscopy has the advantage of being the first analytical method able to resolve quantitatively the chemical information on large samples. Mass spec imaging or other spectroscopies can probably do it, but they are much more complicated and much more difficult to handle.
A big advantage of infrared microscopy is that you don't need to stain. You don't need to manipulate your sample; you just section it, analyze it, and then it's all about reconstruction and mathematical treatments.
Because we don't need to heavily manipulate or prepare the sample, we can have access to all the quantitative chemical information about the sample. This is relevant to every kind of industrial application that is related to the analysis of chemicals. Obviously, a couple of decades ago, the first industry interested in that was the clinical and pharmaceutical industry because the advantage of this microscopy is its spectroscopy; it's a chemical analysis of the sample and it provides a chemical picture of your sample.
In terms of bio-medicine and the pharmaceutical industry, it's obvious that they need to have complete information like this, but I think in the future, other industries will be interested. For example, the food industry could apply it for quality control of food products. They need to know what happens to a nutrient and what happens to food when it's preserved, maintained, stored, transformed, and so on. They need to know the chemical composition of that as part of a quality control process. It may also be useful to the chemistry industry in general, for polymers and things like that. For everything that's in three dimensions, you need to know the organization of a chemical composition.
What do you think will be the next frontiers in the application of 3D-IR?
The next frontier will be that we need to raise this kind of technology and application to a level where it's outside the laboratory. In the laboratory, we can set up the basic tools and methods, but, really, to exploit this kind of technology, we need a much larger facility that is designed specifically for that. For example, large pharma companies that maybe interested could internalize it and set up a laboratory designed for that.
I think this kind of activity is too large and difficult for a single laboratory to handle. We need to find or setup national or international facilities. We need to merge the different skills of all the people who are able to handle this kind of facility. We need to have biologists, spectroscopists, physicians, computer scientists and mathematicians all working together on each new application of 3D chemical imaging by IR spectroscopy.
It's obvious that if I'm developing something for industry, I cannot do everything by myself for the biomedical industry, pharmaceutical industry, food industry, chemistry and so on. We need to design facilities which are designed for the different applications. Then, we can inject in these facilities the common tools we develop for making 3D chemical imaging robust and a routine, I mean all automated means for data acquisition, treatment and visualization.
Many of those projects will involve more industrial participation – are there any industrial partners who are already interested in the potential of this technology?
Since I am involved in histology, my first orientation was bio-medicine. The first grants we get at national/international level, will be for developing this technology for diagnostic methods based on biopsies.
Now, we are implementing some research collaborations with industrial partners in the pharmaceutical industry because we want to perform drug screening at the pre-clinical stage to have a deep impact on pharmaceutical R&D. We want to check the effects of a drug on it's organ’ targets but also on the other organs because we know a drug does not only affect its defined targets, but also the rest of the body.
We are targeting industry for two reasons. The first one is that in terms of a laboratory, to analyze a complete organ, which takes a lot of machine time and human time, we need a very good reason to do it. I don't want to arrange for a Ph.D. student to analyze a mouse brain, if it's only going to show that there is a tumor inside. We need the pharmaceutical industry so that a big challenge can be defined with it. Enhancing drug quality and efficiency for patients is that kind of challenge we are searching for.
The second reason is that development costs for this kind of technology will now be too large for an academic laboratory and because we need to setup facilities, we need to target an industry which is interested in this kind of application. Also, very soon, we will need industry to pay for the continuation of this effort. We can implement the first methods, but if we want to have research and development based on this method, we need industrial partners that are interested in paying for that. It will be servicing industry in this case. Large research institutes can be also the scientific and technological support to develop such platforms if they have the ambition to perform transfer know-hows and machine time to industry.
About Dr Cyril Petibois
Dr. Petibois is head of research group “3D spectro-imaging” at Inserm U1029 in Bordeaux and director of an International Associated Laboratory between Bordeaux and Taipei, Taiwan. He is biophysicist in charge of the development of 3D imaging methods using IR spectroscopy to characterize chemical content changes in tissues and organs (animal models) induced by tumor growth, generative diseases…etc.
He has patented and developed the concept of 3D IR imaging by reconstruction of 2D seriated images with quantitative analysis of chemical contents and their translation into biological metadata. He is implementing IR microscopy as the first hyperspectral imaging tool for 3D histology and pathology, with special interest to applications in the biomedical and pharmaceutical fields.