Before I explain the discovery, I would take a step back and explain an interesting event that takes place in the cancer cells. Normal cells follow a rapid and irreversible process to efficiently eradicate dysfunctional cells.
This is a natural process by which damaged cells commit ‘suicide’. This process is known as apoptosis or programmed cell death.
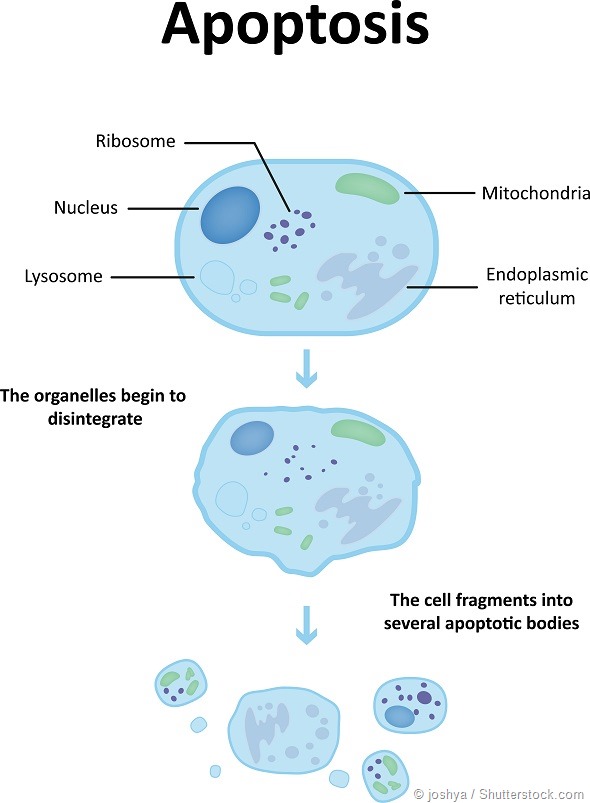
A characteristic of cancer is their unique ability to escape apoptosis. They act ‘smart’ and bypass this normal cellular phenomenon ultimately leading to uncontrolled cellular growth.
Mitochondria, which is the source of energy in the cell, are the primary regulators of cellular suicide. The pioneering work of Otto Warburg (Nobel Prize Winner, 1931) and plethora of other studies led to the inference that functional damage to mitochondria can be linked to cancer.
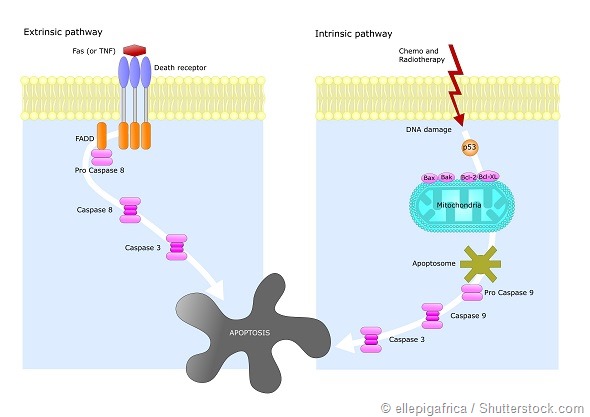
Emerging studies renewed our interest in mitochondrial metabolism as a conceivable target for cancer therapy. To elicit the ‘suicide switch’ back on, our work turns to a highly selective nanotechnology based approach.
To modulate the mitochondrial function, we developed agents that are ‘masked’, impeding the discharge of the active agent during systemic circulation.
The agent acts as an inhibitor of pyruvate dehydrogenase kinase (PDK), an enzyme responsible for reversing the suppression of mitochondria-dependent apoptosis.
These ‘masked’ agents form tiny nanoparticles following a very controlled chemical process. Owing to their tiny size, they are selectively taken up by the ‘leaky’ tumor vasculature.
The ‘masked’ form of the agent then becomes triggered once it gets internalized in a cancer cell and after the effect of certain enzymes abundant in cancer cells. The approach certifies the use of fraction of the drug dosages, dropping overall toxicity while increasing overall efficacy.
Our success is demonstrated in vitro and also in a mouse model. With further development, this approach could offer new hope for cancer patients with much desired improvements in their treatment regimen with affordable treatment options.
How did you make sure that permeation of haloacetates through blood-brain-barrier did not occur?
We are using haloacetates - halogen containing small organic molecules. One of the agents that we are using contain chlorine and is a common by product of chlorination of water.
Interestingly, these molecules based on their polarity, are easily absorbed by body and show high tendency for permeation through blood brain barrier.
This unique characteristic makes their access to the brain rather easy, posing severe neurological and related toxicity.
We used elegant chemistry to develop a ‘camouflaged’ form of these agents, in such a manner that they do not get activated until it finds the desired target (i.e. the cancer cell).
Furthermore, these agents self-assemble into tiny nano-particulates which are selectively taken up by the tumors by their leaky vasculature- a common defect known as ‘enhanced permeability and retention factors’.
Blood-brain-barrier is comprised of tight junction of endothelial cells. Haloacetates being in a protected form and within a nano environment cannot have facile access through the blood brain barrier.
What agent did you use as precursor material and why is this particularly exciting?
Haloacetates are widely known small molecules. Once of the agents, dichloroacetic acid (DCA) is a common by product of chlorination of water.
These agents are inexpensive, not proprietary. Easy access to these molecules will make the further chemistry simple and highly scalable at a reasonable cost, making the nano formulation widely available for cancer patients use.
What techniques were used in your research and which instruments were key to obtaining your results?
We are working on a complex biomedical problem which needs a multidisciplinary approach to solve. My laboratory trains students and postdocs to gain expertize in multiple disciplines of chemistry, biology and engineering.
For this particular project, once the conception of the idea and initial design were made, the compounds were screened computationally to study their interaction with the intracellular target.
In this case, it is a ‘gate keeper’ enzyme in mitochondria known as pyruvate dehydrogenase kinase (PDK). PDK is activated in wide ranges of cancers and significances in the selective inhibition of pyruvate dehydrogenase to suppress mitochondrial apoptosis in cancer cells.
Once the comparative selectivity was established, the syntheses of the prodrugs was undertaken followed by extensive physico-chemical, in vitro and in vivo characterizations.
Dr. Santosh Misra, a postdoctoral researcher in my laboratories conducted the nanoformulation and biological studies using techniques such as probe sonication, microfluidization, PCR, gel electrophoresis, FACS and other biological assays.
Fatemeh Ostadhossein, a graduate student in my laboratories conducted the majority of the physico-chemical characterizations using dynamic light scattering, TEM, NMR, Circular Dichroism etc.
Your research was carried out in rodents, do you think the results will hold true in humans?
Testing in rodent model is a common way to show efficacy and initial success under preclinical environment.
These results indicated both mechanistically and physiologically that these agents can show effectiveness in live animals. Further developments will be required to show non toxicity in preclinical models and efficacy in larger animal models.
What further research is needed before this technology can be used in a clinical setting?
Typically, the regulatory pathway for a new agent to reach clinic could take a long time anything from 7-14 years. However, we may have a solution here.
DCA has a long history of human use, but, the prodrugs (camouflaged form) and the self-assembled nanoformulation does not. Our design allows us to use only GRAS (‘generally recognized as safe’) quality of materials including lipid which is an essential component of human system.
We need an in depth preclinical toxicity assessment to evaluate these agents. For that, mutual talks are in progress with governmental agencies. Efficacy study in large animal model will also be performed in parallel.
Once that is accomplished, a clinical trial could be performed. We anticipate a relatively faster pathway to clinically translate these agents to human.
If successful, what impact could this approach have for cancer patients?
Treatment cost for cancer is sharply increasing. In 2015 alone, more than $40 billion USD was spent on oncotherapy and its supportive care treatments. On an average some of the newly available advanced drugs cost around ~$20K per month.
This sharp increase in the cost of oncology medication is a matter of great concern for the medical community.
Things are even worse in the developing countries. It is therefore of critical importance that we develop new technologies to make treatment options widely available across the community and around the globe.
Since these agents are derived from inexpensive materials and the chemistries performed are relatively straightforward, we envision that the successful outcome of our studies will have significant impact by making cancer therapy widely available for everyone at a fraction of today’s cost.
Where can readers find more information?
They can read our published work here: http://www.nature.com/articles/srep28196
About Prof. Dipanjan Pan
Prof. Dipanjan Pan, PhD, FRSC is a Fellow of Royal Society of Chemistry and an expert in nanotechnology, materials science, molecular imaging and drug delivery.
He is presently the director of Masters in Engineering Program in Bioinstrumentation in the College of Engineering at University of Illinois, an Assistant Professor in Bioengineering, Materials Science and Engineering and Institute of Sustainability in Energy and Environment in University of Illinois, Urbana-Champaign, USA.
He also holds a full-time faculty position with Beckman Institute for Advanced Science and Technology and University of Illinois Cancer Center.
Earlier he was an Assistant Professor in Medicine, Research in Washington University School of Medicine, St Louis. His interdisciplinary research develops next generation personalized nanomedicine through in silico-to-in vivo approach.
Over the years, this work has resulted in numerous high impact publications in peer-reviewed scientific journals, patents, abstracts and received funding support from the NIH, NSF, AHA and other sources.
He is the founder or co-founder of three University-based biotechnology start-ups to translate nanotechnology based solution for human diseases. He serves as an editor for Scientific Reports (Nature Publishing) and also acts as an editorial advisory board member of Molecular Pharmaceutics (ACS).