The Food and Drug Administration (FDA) has approved a device that automatically monitors blood glucose levels and delivers insulin when appropriate, for people aged 14 years and older who have type 1 diabetes.
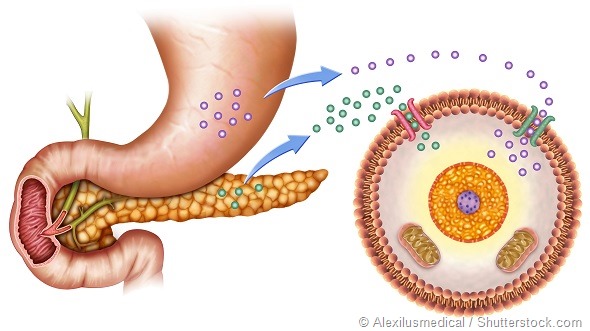
In healthy individuals, the pancreas ensures the supply of a continuous background level of insulin, referred to as basal insulin. In people with type 1 diabetes, the pancreas either fails to produce this insulin or its ability to do so is impaired.
To maintain a steady blood sugar level, people with type 1 diabetes have to continuously monitor themselves throughout the day, usually by drawing a small drop of blood from the fingertip.
They self-administer insulin several times a day using a pump or needle. However, the amount of insulin required varies depending on a number of factors such as what the person ate earlier, whether they have exercised or even whether they are feeling stressed.
This means that the blood sugar maintenance targets that need to be met in order to reduce the risk of complications such as organ damage or hypoglycemic shock is only met by 28% of teens and young adults, according to a 2014 article published in Diabetes Care.
Medtronic’s MiniMed 670G hybrid closed looped system, which is often referred to as an “artificial pancreas,” automatically administers or withholds insulin in response to blood glucose measurements, which it takes every five minutes.
This first-of-its-kind technology can provide people with type 1 diabetes greater freedom to live their lives without having to consistently and manually monitor baseline glucose levels and administer insulin,”
Jeffrey Shuren, director of the FDA’s Center for Devices and Radiological Health.
The system is made up of a sensor that measures blood sugar under the skin; an insulin pump; an infusion patch that delivers insulin from the pump via a catheter inserted into the skin and a computer chip that uses data to optimize insulin delivery by the minute.
The FDA evaluated data for the device from a clinical trial that involved 123 participants with type 1 diabetes. The results showed that the system significantly reduced the average blood glucose level over three months, with the most improvement seen among people who had the highest blood glucose levels at baseline.
Alberto Gutierrez, also from the FDA’s Center for Devices and Radiological Health says: “As part of our commitment to improving diabetes care, the FDA worked interactively with Medtronic from the earliest stages of development to assist in making this technology available to people with type 1 diabetes as quickly as possible.”
As part of the approval, the FDA needs a post-market study to assess how the system performs in real-world scenarios. So far, the device has only been approved for use by people aged 14 years and older, but Medtronic is now carrying out further research to assess how safe and effective the system is for children aged 7 to 13 years.