Oct 20 2016
The Case for Quality, a collaboration between FDA, the medical device industry, healthcare providers and quality experts, released its first report on the feasibility and effectiveness of using standardized medical device performance data and analytic techniques to help hospitals better compare and evaluate product quality.
"The goal is to help hospitals make better purchase decisions and potentially improve patient outcomes,” said Joanna Engelke, Senior Vice President, Global Quality & Regulatory, Boston Scientific, and a member of the Case for Quality Product Quality Outcomes Analytics Working Group, which includes representatives from device manufacturers, healthcare providers, FDA and hospital Value Analysis Committees (VACs).
Case for Quality is a multi-year initiative launched by FDA to develop best practices, standards, tools and metrics that can be used by both the agency and industry to improve product and manufacturing quality in ways that go beyond compliance with regulatory requirements. The effort is being coordinated by the Medical Device Innovation Consortium (MDIC), the first public-private partnership between FDA, industry and other collaborators, created with the sole purpose of advancing medical device regulatory science.
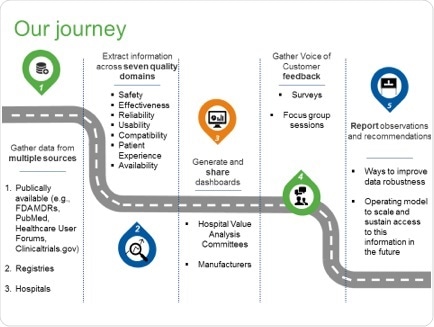
In developing its report, the Case for Quality Product Quality Analytics Working Group conducted a pilot with seven hospitals focused on knee implants and implantable cardioverter defibrillators.
Data was collected from several publicly available data sources including the MAUDE database, and the manufacturers themselves, around seven quality parameters: safety, effectiveness, reliability, usability, compatibility, patient experience and availability.
This data was then analyzed to calculate key performance indicators, which was presented to VACs via four quality dashboards that contained an overview and rankings by data source, manufacturer and product. The VACs provided feedback through surveys and focus groups.
“This is the first step toward applying a standardized model of data and techniques to device performance,” said Engelke. “More work remains ahead to mitigate data bias, ensure accurate interpretation and use of the data and independent management of it. We look forward to undertaking this work, which may lead to better tools for monitoring device performance—and, ultimately, better patient outcomes.”
The full report is available at the MDIC’s Resource Center for Sustained Quality page here: http://mdic.org/cfq/resource-center-for-sustained-quality/. MDIC’s reports on Quality Metrics and Maturity Model Research from two of its other working groups can also be found there.
The Case for Quality was established after an in-depth review and analysis of device quality data gathered between 2001 and 2011. It revealed that companies that went beyond minimum compliance requirements and managed risk by driving quality across their entire organization had fewer device complaints and investigations. Those with an established quality culture were also able to accelerate device design, innovation and market introduction by avoiding quality failures.
“Case for Quality is setting a cultural shift in motion to focus the medical device ecosystem on sustained product quality that enhances patient safety and outcomes,” said Bill Murray, president and CEO, MDIC. “We’re making great progress and we expect even greater participation from the medtech ecosystem in 2017.”
The next Case for Quality Public Forum is scheduled for October 26, 2016 in Washington, DC.