Jan 3 2017
Researchers affiliated with the Kawasaki INnovation Gateway at SKYFRONT, have successfully generated the first ever non-human primate X-SCID models by using two genome editing techniques. The findings were published in Cell Stem Cell, July 2016.
Researchers from Japan and the US have generated the first ever non-human primate models for X-linked severe combined immunodeficiency using state-of-the-art genome editing techniques.
Further information about science and technology projects at Kawasaki City is available in the Kawasaki SkyFront iNewsletter that highlights research being conducted by scientists and industries affiliated with Kawasaki INnovation Gateway at SKYFRONT (KING SKYFRONT)—the City’s flagship science and technology hub launched in 2013 to focus on open innovation in the life sciences and environment.
December issue of Kawasaki SkyFront iNewsletter
http://inewsletter-king-skyfront.jp/en/
While many insights are gained into diseases and genetic disorders from rodent models, there is a pressing need to find models that can more accurately represent disease progression in the human body, such as non-human primates. The latest advances in genome editing technology are opening doors to generating non-human primate models for studying specific genetic disorders, such as X-linked severe combined immunodeficiency (X-SCID).
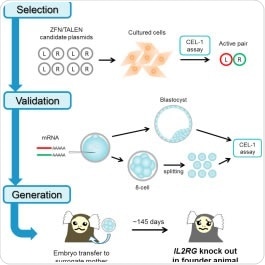
X-SCID patients do not produce enough T-cells and natural killer cells to tackle infections. The condition stems from a defective gene, IL2-RG, and scientists are keen to know more about the disorder and how to improve treatment. Now, Erika Sasaki at the Central Institute for Experimental Animals, Kawasaki, and co-workers across Japan and the US, have successfully generated the first ever non-human primate X-SCID models by using two genome editing techniques – zinc-finger nucleases (ZFNs) and transcription activator-like effector nucleases (TALENs).
The team worked with marmoset monkeys because they are easy to handle and have a small body size perfect for testing small amount of new drugs in preclinical trials. They screened the most effective ZFNs to knock-out IL2-RG gene among the 16 pairs of candidate ZFNs cultured cells. They then injected the selected ZFNs into 1 cell stage marmoset embryos and performed a specialized screening test to check for, and avoid, mosaicism (if the models carrying the mutant gene in the all of the tissues in the body or not).
The researchers then transferred the embryos to surrogate mothers. Of the 5 ZFN and 4 TALEN baby marmosets that were born, three have since survived to adulthood and represent the first group of X-SCID non-human primate models ever created.
Further tests showed that the IL2-RG knockout marmoset models exhibited strong phenotypic similarities to human patients with X-SCID. Sasaki’s team are confident that their work paves the way for the creation of multiple gene knock-out models for specific diseases and genetic disorders.
King SkyFront -Kawasaki, innovation and a gateway to the world-