Jan 10 2017
Researchers at Okayama University have uncovered a potential new therapeutic target for invasive bladder cancer. The GTP hydrolysing enzyme (GTPase), called Dynamin2, facilitates the rapid invasion of cancer into surrounding tissues; inhibiting its activity could limit the progression of bladder cancer.
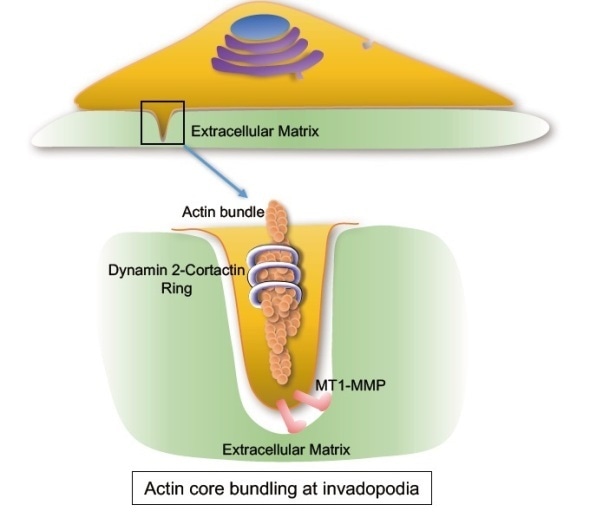
Graphical abstract of Dynamin2 function at the invadopodia. Dynamin2 and cortactin forms a “ring” shaped complex required for the formation of “F-actin core” of invadopodia. Dynamin2 may also be involved in trafficking of MT1-MMP protease to the invadopodia to degradate extracellular matrix.
Bladder cancer is one of the most common forms of urological cancer, and removal of the bladder remains the primary method for treating the condition. However, the surgery impacts heavily on patients’ quality of life and places considerable burden on healthcare services to care for those in recovery. Alternative treatment methods are sought to reduce the need for such drastic surgery.
Many cancers, including bladder cancer, progress via the formation of ‘invadopodia’ – protrusions made from bundles of F-actin that promote cell invasion by degrading the extracellular matrix of healthy cells. A family of GTPase known as Dynamin have been implicated in cancer progression and invadopodia, but the precise mechanisms involved are unclear.
With this in mind, Tetsuya Takeda and co-workers at Okayama University investigated the role of three types of Dynamin (1, 2, & 3) in invasive bladder cancer. They found that all the three Dynamin isoforms were expressed in bladder cancer cell lines, but that only Dynamin2 localized to the invadopodia.
Further examination showed that the proline/arginine-rich domain of Dynamin2 is vital to the correct formation of invadopodia. Takeda’s team found that Dynamin2 interacts with cortactin – a protein that can trigger the rearrangement of F-actin filaments – and that these interactions are crucial for the stable formation of invadopodia. When the researchers inhibited Dynamin2 in cancer cell lines, they observed severe defects in invadopodia formation and suppression of cancer cell invasive activity.
While further investigations are needed to clarify the mechanisms involved in invadopodia formation, these results indicate that Dynamin2 may provide a valuable therapeutic target to reduce the growth and invasiveness of bladder cancer.
Background
Cancer cell invasion
Cancer cells use invadopodia – protrusions on the cell membrane created by bundles of F-actin (linear polymer microfilaments) – to degrade surrounding tissues (invasion). Invadopodia are also implicated in a cancer’s ability to metastasize. Scientists believe that, if they can work out how to prevent invadopodia formation in different cancers, the progression and invasiveness of the cancer could be better contained and more easily treated, thus reducing the need for surgery and more aggressive treatments.
The Dynamin family proteins play a key role in the absorption of hormones and proteins by healthy cells. However, Dynamin2 has also been implicated in cancer cell migration and metastatic invasion because of its ability to stabilize actin-based structures. This led Tetsuya Takeda and his team to hypothesize that Dynamin2 may be involved in the formation of F-actin-based invadopodia. Their results show that Dynamin2 is a vital component of correct invadopodia formation in invasive bladder cancer. More precisely, interactions between Dynamin2 and the cortactin protein create more stable F-actin structures, encouraging invadopodia growth.
Implications of the current study
The discovery that Dynamin2 plays a crucial role in the correct formation of invadopodia could inform future treatments for bladder cancer. Takeda’s team successfully inhibited Dynamin2 activity in cancer cell lines, leading to defective invadopodia and the suppression of the cancer’s invasive properties. Following further investigations, the same principle could be carried forward to create a new bladder cancer therapy that targets Dynamin2.