Putting patients at the centre of clinical trial research is perhaps something that goes without saying. At the Clinical Innovation and Partnering World congress in London last week, this was much discussed, along with the need to increase efforts into engaging patients to take part in clinical trials, and the fact that patients are sometimes suspicious of research because it is not explained enough and patient engagement is poor.
The issue of poor patient engagement in clinical trials was introduced by Jonathan Sheffield, Chief Executive Officer, National Institute for Health Research, who mentioned the difficulty in finding and subtyping patients for trials. This is an unnecessary challenge, as patients can do a lot of phenotyping themselves, they just need to be asked. However, where the problem lies, is finding a way to engage them.

Hilde Vanaken, Director R&D Operations Innovation, Janssen continued with the idea that there is poor support for patients in clinical trials. Janssen provides a solution to this through a smart technology program called eMeds, which aims to transform clinical trial medication and data management, and enable personalized engagement and support for patients.
The concept of innovation in the hospital in order to put the patients’ needs at the heart of all delivery was demonstrated by Joana Claverol, Clinical Research Unit Manager at Sant Joan de Deu Barcelona Children’s hospital. Here they have made adjustments to equipment and procedures in order to improve comfort and reduce anxiety for children in hospital. One example of this is a low cost Google CardBoard VR system which is used to distract children from the fear and pain caused by hospital procedures.
Guy Yeoman, VP Patient Centricity, Global Medical Affairs, from AstraZeneca asked the thought-provoking question:
What happens when you stop just thinking about the patient, and starting working WITH the patient?”
Here he introduced the PaCe portal, which puts patients first. With this system, by working with patients, it will be possible to deliver research that patients want to participate in and medicines that they want to receive.
Guy was then joined by a panel including Bert Hartog (Director Clinical R and D, R&D Operations – Innovation leader, Johnson and Johnson), who stated that:
Patients shouldn’t feel like they have missed out on treatment because they didn’t know trials were running”
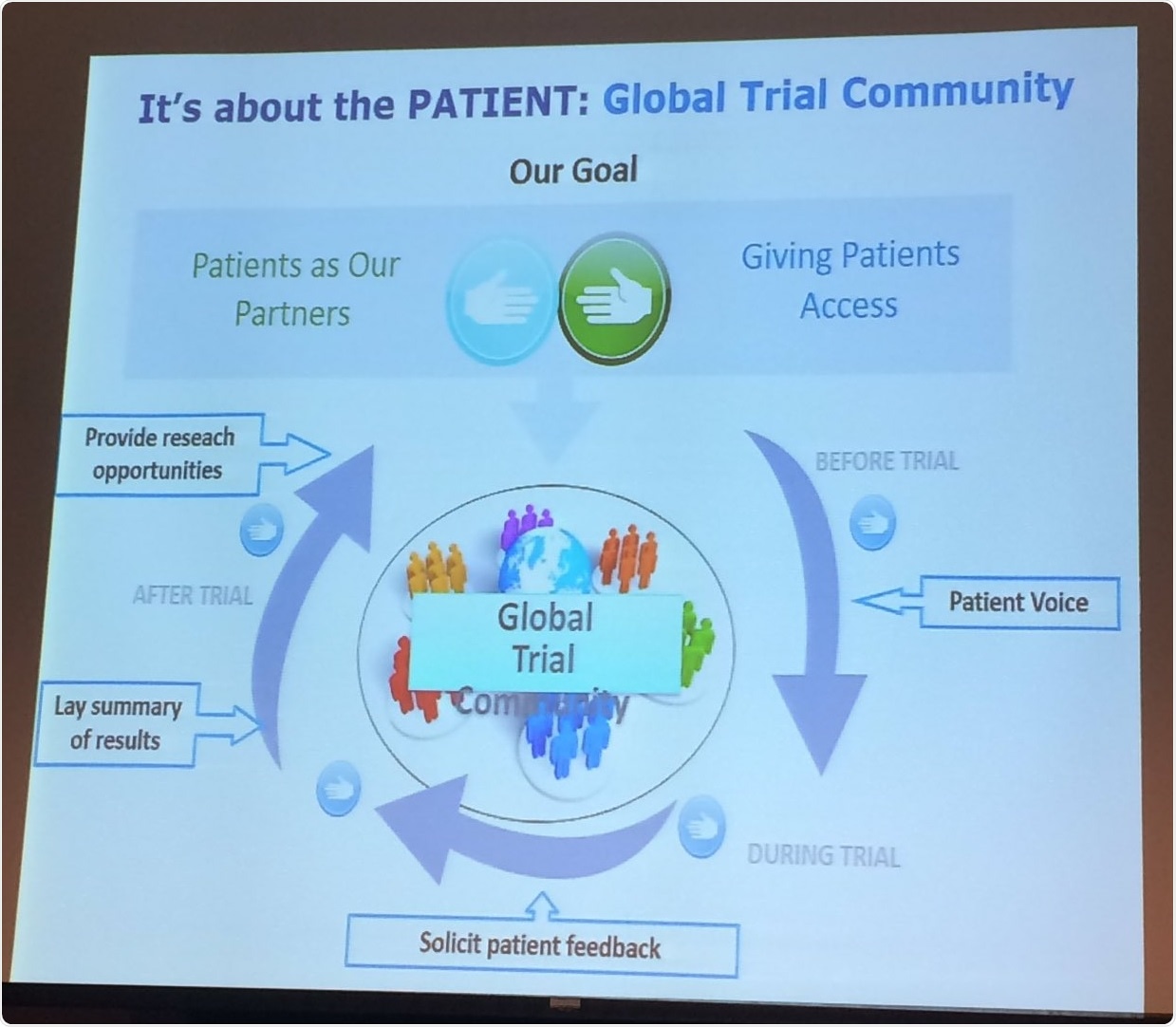
Bert also adds that it would be a disservice to patients to not fully inform them of clinical trial opportunities, and even suggests the provision of a trial community. These statements reiterate the importance of informing and engaging relevant patients about current trials that they would be suitable for and may benefit from.
Simon Ridley, Director of Research from Myeloma UK also supported this point in the panel discussion, and later in his talk highlighted that the focus should be on trials that are the most important in terms of patient benefit rather than academic achievement.
The afternoon was occupied with talks on developing a successful outsourcing strategy. This included a presentation from Renaud Burrer, R&D Manager at Histalim, who discussed the importance of choosing the right central lab for improvement and innovation of clinical trials.
Histalim are a histology service provider and specialists in immunohistochemistry and image analysis. By integrating new methods concerning histology and re-designing them so that they produce high quality results, Histalim can help research objectives of clinical trials to be met.

The day drew to a close with some final statements from Adama Ibrahim Senior Clinical Operations Lead at Biogen Idec:
We need to continue to collaborate… We need to advocate patient engagement”
With this in mind, it is clear that patient centricity in clinical trials is essential and moving forward this should be at the core of all clinical research.
By making the most out of digital technologies, working with the patient and listening to their needs, it will be possible to engage and recruit patients that will gain the most benefit by taking part in the trials.