Researchers at the Cedars-Sinai and NeuroVision Imaging LLC have made it possible to identify the pathological markers of Alzheimer’s using retinal scans. These scans are non- invasive and could be performed routinely in future to detect this dreaded form of degenerative brain disease. The paper was published online in the JCI Insight on 17th of August.
Beta amyloid protein is one of the hallmark features of Alzheimer’s where deposits of this protein are found in the brain. Usually positron emission tomography, or PET scans of the brain and testing the cerebrospinal fluid taking from the base of the spine are used to detect this protein in Alzheimer’s. These are expensive as well as inconvenient. This makes them impractical for use as a screening tool for large populations. The team of researchers used retinal scans that are relatively routine and inexpensive and easy to perform to do the same job. The retinal images could mirror the brain image.
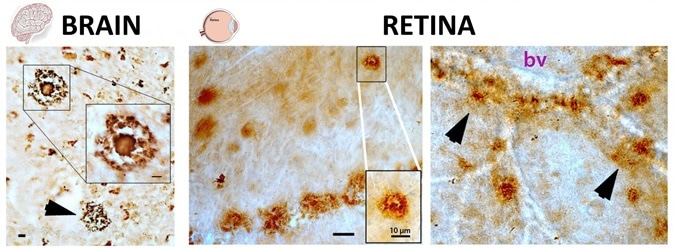
Pathological hallmarks of Alzheimer's disease, beta-amyloid plaques (brown spots), as seen both in brain and retinal tissues from deceased patients, as described in the Aug. 17 edition of Journal of Clinical Investigation Insight (Koronyo et al. 2017). The structures of these plaques in the brain and retina are very similar (classical and vascular-associated deposits; bv = blood vessel), suggesting that the retina faithfully represent the brain disease. Image Credit: Image adapted from JCI Insight. 2017; 2(16):e93621. doi:10.1172/jci.insight.93621
Dr. Maya Koronyo-Hamaoui, an associate professor of Neurosurgery and Biomedical Sciences and a research scientist at the Maxine Dunitz Neurosurgical Institute at Cedars-Sinai, and a co-founder, inventor and scientist at NeuroVision is one of the lead authors of this study. She said this is a pioneer study that could detect the beta amyloid proteins with accuracy in living patients using high resolution retinal scans. The study plan was robust she explained with comparison of the beta amyloid deposits in the retina of Alzheimer's patients with matched control participants to rule out any bias. The analysis also compared respective brain and retinal pathologies to be further sure. She said that this study paves the way to make retinal imaging a “surrogate biomarker” that could be used to detect Alzheimer’s and also monitor its progress over time. A biomarker is a biological test that could detect a disease or ailment.
Dr. Keith L. Black, chairman of NeuroVision, chair of the Department of Neurosurgery and director of the Maxine Dunitz Neurosurgical Institute at Cedars-Sinai explained that Alzheimer’s can begin around a decade or two before the symptoms of memory loss and cognitive impairment start to become evident. Early detection by routine screening thus becomes “crucial” to tackle this debilitating condition by starting treatment early. The retina is an outgrowth of the brain when the fetus develops in the womb and thus it has several features that mirror the brain he explained. Thus a look at the retina could offer a picture of the brain itself.
Yosef Koronyo, a research associate at Cedars-Sinai and a scientist and inventor at NeuroVision who is the first author of this study said that in 2010 this team had come up with a study that showed that Alzheimer’s-specific plaques - a pathological picture within the Alzheimer’s affected brain – could be seen on the retina. A modified ophthalmic device could be used to detect these plaques in experimental mice models’ eyes he said. This study is a culmination of those findings Koronyo added. Their early findings prompted the team to initiate human clinical trials in the United States and Australia to check if the detection and quantification of the beta-amyloid plaques in patients with the disease actually worked.
In this new clinical trial the researchers looked at 16 patients and tried to identify beta-amyloid in the eye using autofluorescence imaging. This was added to the analysis of donated eyes and brains from 37 deceased patients who did (23 patients) or did not (14 controls) have Alzheimer’s.
Results showed that there was a 4.7-fold increase in retinal plaque presence in patients with Alzheimer’s when compared to controls who did not have Alzheimer’s. The exact locations of the amyloid plaques were also mapped in the participant retina and brain by the researchers. They identified plaque clusters called “hot spots” in the retina that contained the most toxic forms of beta-amyloid proteins and these were placed in the top outer regions of the retina. The beta amyloids were studied in details under the electron microscopes. Electron microscopy showed that these amyloids occurred in protofibrils and fibril assemblies.
Automated calculation of the quantity of retinal amyloid deposits was also done and this was marked with the retinal amyloid index (RAI). There was a 2.1-fold increase in patients with Alzheimer’s in terms of quantity of the beta amyloid plaques.
The study was funded by one National Institutes of Health/National Institute on Aging (NIA) award (AG044897; The Saban Family Foundation and The Marciano Family Foundation (Koronyo-Hamaoui).