An interview with Dr Michael Graz and Dr Graham Dixon, conducted by Alina Shrourou, BSc
How prevalent is Cystic Fibrosis and how is it diagnosed?
There are about 77,000 people known to have cystic fibrosis. That's from the various cystic fibrosis registries available globally. The World Health Organization suggests that this number may be low, because there's no reporting on cystic fibrosis from the developing world. The accepted number, at the moment, is about 80,000. That's the one that is used for most of the work that's being done on cystic fibrosis.
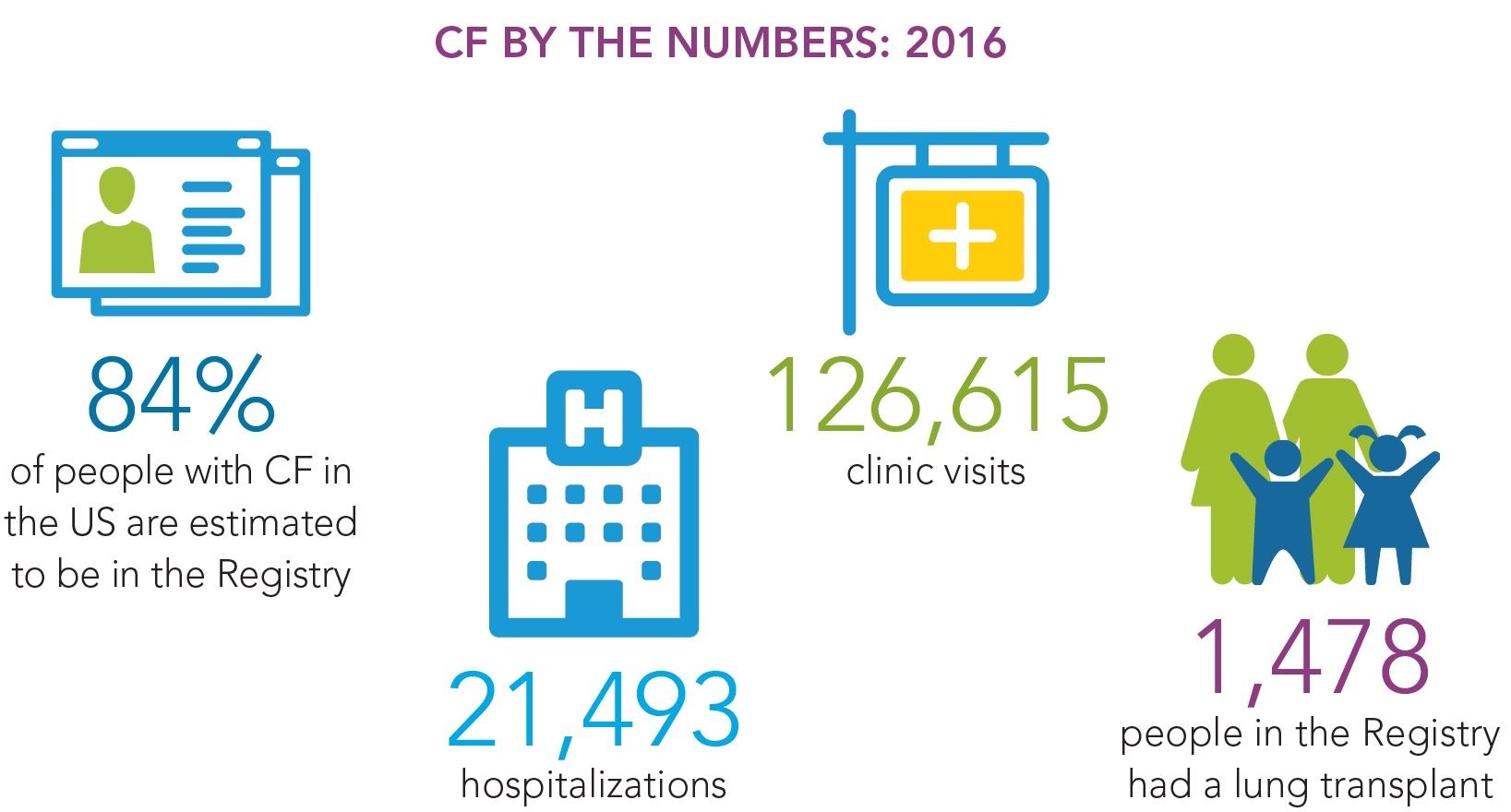
Credit: Cystic Fibrosis Foundation Registry Highlights 2016
Diagnosis is mainly done by newborn screening. Although, there is late diagnosis for adolescents and adults that were born before the newborn screening came in. This late diagnosis is usually used for patients that have only been exhibiting mild forms of the symptoms, and it’s only when they start developing complications later in life that cystic fibrosis is diagnosed.
Diagnosis also varies from country to country, with various countries having different approaches on how to do screening. In some countries, newborn screening is performed on every child that is born, whereas in some countries, like the UK for example, it is based on parental choice.
Why is Cystic Fibrosis under diagnosed in developing countries?
For many years, cystic fibrosis was thought to be a disease of Caucasians. It's only been reported for the last couple of years, that the mutation does also occur in other race groups.
Up until now, in mostly non-Caucasian countries, there was no screening for cystic fibrosis because it was not seen as a disease of any note. It's not because of any specific healthcare standards, but because it wasn't thought to be prevalent in those countries.
Can any other complications occur in individuals diagnosed with CF?
Apart from the effects on the lung, you can get digestive issues. Cystic fibrosis also affects the functioning of the kidneys and pancreas.
Some of the antibiotics that are used have known side effects such as hearing loss or potentially kidney damage over and above the complications just found in the patient, and yet the patient population is willing to use those treatments because the benefit outweighs the risk.
What are the current challenges in Cystic Fibrosis research?
You can put cystic fibrosis research in two sections. The first is the treatment of the actual disease state, which is looking at disease-modifying agents, of which there is one effective one available, but for only a small subset of the cystic fibrosis population. There is a lot of focus on how you can potentially correct the mutation.
The other type of research is about treating the symptoms and comorbidities. Both have their own challenges.
There are several different mutations that affect small percentages of the population. Many of the drugs have been developed against a certain mutation, so they don't have a whole spectrum of activity. That's why you see, in treatment, many companies are now even up to triple combinations of drugs to try and get around that. That creates challenges.
If you're trying to deliver three drugs to the same place, they must all have the same pharmacokinetics, etc. The combinations have to be carefully developed and put together. To find a disease-modifying drug that would be applicable to all the population would be a great breakthrough.
Why is a combined approach often needed with CF therapies? Is the condition facing antimicrobial resistance?
With most areas of infection, not just infection in cystic fibrosis, multiple therapies are starting to become regularly indicated. This is to overcome the potential for antimicrobial resistance or to overcome the fact that there is such a prevalence of antimicrobial resistance that you need multiple prongs of attack in order to be able to clear an infection.
Because of the chronic nature of the infections in cystic fibrosis patients, often the only way that these infections can be managed is with very potent antibiotics - antibiotics, which in other cases would be used only as a last resort. These are given in some form of combination in order to give the patients the best quality of life.
What is antimicrobial resistance and why is it becoming more of an issue?
Antimicrobial resistance is the result of an evolutionary mechanism in bacteria. That mechanism allows bacteria to survive in difficult environments. For example, bacteria that can grow at the bottom of the ocean next to thermal vents, or in ice, or survive in the saline environment in the sea.
The bacteria develop mechanisms to cope with their environment. Antimicrobial resistance is effectively just a mechanism bacteria use to cope with the stress, which is caused by the antibiotics that we use.
Resistance has been there ever since bacteria have been around. If you look at the data from ancient bacteria that were taken from underneath the ice cap, these already exhibited some of the enzymes that confer antimicrobial resistance, before they'd ever been exposed to what modern medicine calls an antibiotic.
Antimicrobial resistance is considered to be either intrinsic or extrinsic. If it's intrinsic, the mechanism is inherent in the bacteria, usually by an enzyme that they've developed over time because of this evolutionary stress. If extrinsic, it means that that they effectively inherit the genetic material necessary in order to make them resistant.
Transfer of the genetic information to confer resistance is a natural phenomenon, that makes microbes, viruses and fungi, become very difficult to deal with. This is especially the case for bacteria, which seem to have the most ability to become resistant. When antimicrobial resistance is transfered between species, this becomes a significant problem if the resistance transfers to a bacterium that is already resistant to most of the other classes of antibiotics.
Even though the problem of antimicrobial resistance is large within its own right, the situation is made worse due to the decrease in R&D performed in big pharma because anti-infectives are often the poor relation compared to some of the other therapeutic areas. When they look at the business cases for disease indications such as oncology, diabetes, etc, these look much more attractive in a commercial sense.
What is the importance of events, like the North American Cystic Fibrosis Conference, to you and the rest of the CF research community?
As in any therapeutic area, there's a great advantage in getting people from diverse backgrounds together for a common goal. This meeting is probably the premier research meeting in the world in cystic fibrosis. It’s a great opportunity to discuss and share the challenges, and of course to network too.
I think it's particularly important, from our perspective as a relatively small biotech situated in Wales, to try and get known in the US. It's particularly the case in cystic fibrosis because there is a very strong Cystic Fibrosis Foundation, which is actively involved in the US, both in terms of research and development, but also in terms of the patients.
For us to be able to go and present a poster, to be able to engage with the US scientists, medical professionals and the Cystic Fibrosis Foundation is a great opportunity for us.
Please outline the information you will be presenting at the North American Cystic Fibrosis Conference next month.
We are presenting a poster as well as also doing an oral presentation as a short summary. It's about the symptomatic treatment of cystic fibrosis. We're looking at trying to help with the infections that occur in these patients. One in particular, Pseudomonas aeruginosa - a pathogen that creates chronic infection.
Credit: Kateryna Kon/Shutterstock.com
Pseudomonas aeruginosa survives for a long time because it produces what's known as a biofilm. The biofilm is an extracellular matrix, which protects it from the environment. In some cases, the environment can be “trying to treat the bacteria with antibiotics”, to kill it.
We will be presenting our information on a novel mode of action, which aims to sensitize the bacteria to the antibiotic to allow it to be killed. It breaks down this biofilm, and that then allows the antibiotic to get to the bacterium and do what it's supposed to do in killing the bacterium.
What are quorum sensing inhibitors and how do they work? Can bacteria become resistant to them?
Quorum sensing is the means by which bacteria communicate using chemical signals. It was first described in the late '90s by Bonnie Bassler, who wrote the seminal paper on Vibrio fischeri, where they showed how you could get them to luminesce in the presence or absence of signals.
Once that was established that bacteria can not only communicate but can switch on or off specific genetic programs based on this communication, people have been postulating, "Well, if you can interfere with that signal somehow, then you can switch off these genetic programs."
In our case, our quorum sensing inhibitor targets a very specific quorum sensing mechanism. It's the type 2 secretion mechanism in Pseudomonas, which is based on homoserine lactone, which is the signaling molecule.
The signaling molecule switches on the virulence factors in the bacterium. These virulence factors are things like the production of flagella so that the bacteria can move to the site of infection. As well as the production of the extracellular polysaccharide that they then protect themselves with. Also, the production of some of the disease-causing agents such as a toxin, and quite specifically to cystic fibrosis, Pseudomonas also produces what is called the cystic fibrosis inhibition factor, often just called cif. The expression of this factor is also controlled by this signaling mechanism.
What you want to do is to be able to switch off that mechanism. You can do that in a number of ways. You can either try and bind the signaling molecules, which, considering that they swarm through the environment, is the harder thing to do. Alternatively, you can bind the actual receptors for those molecules.
That then means the signal can't get to the point that it needs to get to, to trigger the next event. Or you might even block the mechanism much further into the cell at intracellular signaling or even at the genetic level.
All of these approaches have been looked at. Ours, quite specifically, targets the outer edge of the cell, where we interfere with the receptors of the molecule.
It would not be right of me to say that bacteria will never develop resistance to this, but because you’re not actually exerting stress on the bacterium, but rather just preventing it from receiving a certain signal, you're much less likely to cause a mutation in that system.
How else is Neem Biotech helping to tackle antimicrobial resistance?
Most of the creative science in antimicrobials is now done by biotech companies, such as Neem Biotech, and then passed on to the big pharma when it's been taken to a certain stage, de-risked, where they can take it forward through to launch.
Our approach is much broader with our collaborations within alliances, for example with the BEAM Alliance - a European-focused group of small biotech companies that are all working in the space of antimicrobial development, to try and get a voice for the small company to be heard at a political level. BEAM has been recognized by the WHO as an alliance, and we’re pleased to announce that were invited to the meeting of the G20, where the G20 in Hamburg make their comments on the battle against antimicrobial resistance. Being involved in that and getting a voice out there, is just as important as actually working on the scientific issue.
Then also, we have our collaborations with international experts in the field of antimicrobial resistance, where we do joint research, or we have world-renowned people advising us so that we ensure that the approach that we're taking encompasses as many of the scientific challenges that we may be facing.
We also engage with patient groups. Here in the UK, the Cystic Fibrosis Trust; in Germany with Mukoviszidose Institut; and whilst Graham is in the US for the North American Cystic Fibrosis Conference, we are also going to start talking to the Cystic Fibrosis Foundation. Involving the individuals that you're developing your treatment for is important, because there is very much a need for them and their challenges to be considered when doing our research.
Are there ways that we can slow down the growing issue of antimicrobial resistance?
The most important activity in slowing down antimicrobial resistance is antibiotic stewardship. Antibiotics must only be used when there's no other intervention that you can use to stop, halt, prevent or treat the infection. Thankfully, this is being pushed worldwide.
Unfortunately, sometimes the other processes that are suggested to minimize or to advocate stewardship are more expensive than the antibiotic. One of the biggest killers in the developing world are infections that come from poor hygiene and sanitary conditions. It's unfortunately more expensive to produce clean water than it is to give someone a basic antibiotic.
Stewardship is a great idea and is being applied extensively in the developing world. We can already see very significant decreases in the prescription of antibiotics here in the UK, it's been quite significant over the last couple of years. In Germany, there are also national programs around stewardship. Furthermore, there have been a number of proposals at both national and global level around what can be done.
The development of rapid diagnostics, obviously, is important, because if you can define the bacterium that's causing the problem and choose the right antibiotic from the outset, rather than just empirically hoping that you'll hit the bacterium, this will help to minimize the generation of resistance.
Can we keep up with antimicrobial resistance? What do you think the future holds?
People are putting a lot of effort into novel approaches, including vaccines, new antibiotics, new mechanisms, those are all things that can help. The more alternative approaches that you have, the less you're dependent on antibiotics as a last resort, which then generates the resistance. If you can minimize the usage and dependence on one class of antibiotics, then they will last longer before resistance becomes a major problem.
As previously mentioned, a lot of the work now is going on in small biotech companies, so more funding is becoming available for these companies to work on antimicrobial resistance.
As long as those companies and basic science continue, I think we can keep track with the resistance. By definition, every time you bring out a new class of antibiotic, it shouldn't have resistance already there in the bacterial population. We just need to keep coming up with new ways and new classes and alternative approaches.
Where can readers find more information?
For more information about Neem Biotech: www.neembiotech.com
About Dr Michael Graz 
Dr Michael Graz is Managing Director of Neem Biotech. With PhDs in cell biology and rational drug design from the University of the Witwatersrand and the Nelson Mandela Metropolitan University respectively, Michael’s career has included lectureships, consultancy and interim management roles in the life sciences in South Africa, the UK and the USA.
In the recent past he has held the role of Managing Director in an diagnostic enzyme production SME and, since 2012, at Neem Biotech, an R&D biotechnology SME.
An entrepreneur at heart, he previously led a successful aquaculture startup venture in Chile. Watching the farm and processing facility being built and taking shape into a commercially viable organisation comes high up on Michael’s list of career highlights.
About Dr Graham Dixon 
Dr Graham Dixon is Neem Biotech’s Chief Operating Officer and Head of R&D for the Zaluvida Group. He obtained a PhD in biochemistry at Swansea University and has spent over 25 years in Big Pharma, VC funded and publicly listed biotechnology companies.
As Chief Scientific Officer, Graham has led over ten positive proof of concept programmes in humans and been a part of several new drug approval programmes in biotechnology companies as diverse as Onexo, Sensorion, Addex Therapeutics, Galapagos, Entomed and F2G.
In Graham’s experience, every successful programme that takes a molecule from an idea through to its embodied form in the clinic is another career highlight.