Feb 12 2018
The in vitro diagnostic (IVD) testing market is one of the fastest-growing segments within the medtech industry. The growing number of pipeline products will continue to increase as a focus on personalized medicine becomes the norm, according to GlobalData, a leading data and analytics company.
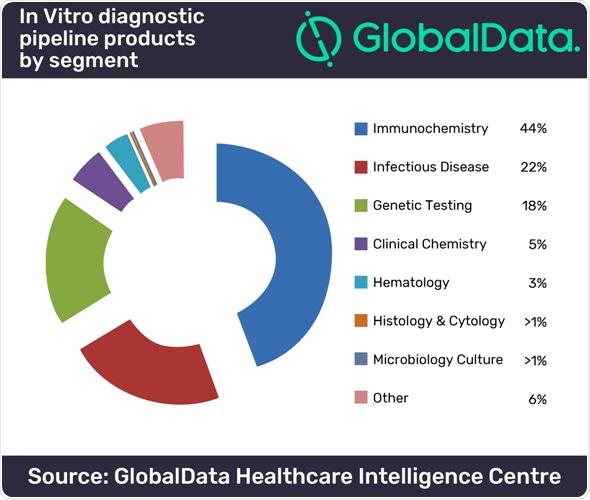
Currently, there are an estimated 10,471 pipeline products for IVD, of which over 60% are active and in development, which is in stark contrast to the second-largest segment in pipeline products, cardiovascular, which only boasts 2,093 pipeline products.
Atif Nawaz, Healthcare Analyst at GlobalData, comments:
The main reason the IVD market is the focus of R&D for most market leaders is due to the fact that IVD spans most applications within the medtech industry. There are a variety of biomarkers indicating illness and disease, which makes IVD testing essential, and this in turn leads to a breadth and depth of IVD products.
Of the IVD pipeline products, 44% are immunochemistry products. Immunochemistry includes a vast array of biomarkers, from cardiac markers to female fertility tests. However, a main component of these pipeline products aims to increase efficiency and streamline test results at the point of care.
Point-of-care (POC) products are essential in detecting and measuring the expression of a specific biomarker linked to disease in order to ascertain how patients respond to particular treatments. POC tests can be utilized to rapidly detect cancer, viral infections, and myocardial infarctions, in addition to numerous other diseases. These tests can be carried out at primary care sites in order to provide timely information to enable rapid decisions from physicians. Likewise, for infectious disease and genetic testing, the move to rapid tests is evident in clinical trials and the way the industry is shaping as a whole.
Nawaz concludes:
As the IVD industry grows at a steady rate, there will be more innovative solutions required to meet the growing need for more accurate and faster results. Patients and physicians alike are demanding effective and streamlined tests to better determine disease and illness, in order to increase the quality of health received and provided, respectively. Manufacturers are frantically trying to innovate to carve out market share for themselves as the demand for IVD testing grows.