Red meat and eggs feed gut bacteria that make TMAO—a molecule now tied to stroke, Alzheimer’s, and heart failure. This new review reveals how diet, probiotics, and even statins could help stop it in its tracks.
 Review: Trimethylamine-N-Oxide (TMAO) as a Rising-Star Metabolite: Implications for Human Health. Image Credit: zizou7 / Shutterstock
Review: Trimethylamine-N-Oxide (TMAO) as a Rising-Star Metabolite: Implications for Human Health. Image Credit: zizou7 / Shutterstock
What you feed your gut microbiota can influence your risk of heart disease and neurodegeneration. Recent research has highlighted the role of trimethylamine-N-oxide (TMAO), a gut microbiota-derived metabolite, as a key biomarker in health, metabolism, and disease.
In a recent review published in the journal Metabolites, a team of scientists in Italy investigated the role of diet and gut bacteria in the biosynthesis of TMAO, as well as the impact of this metabolite on the risk of neurodegenerative and cardiovascular diseases.
Gut-microbiome metabolites
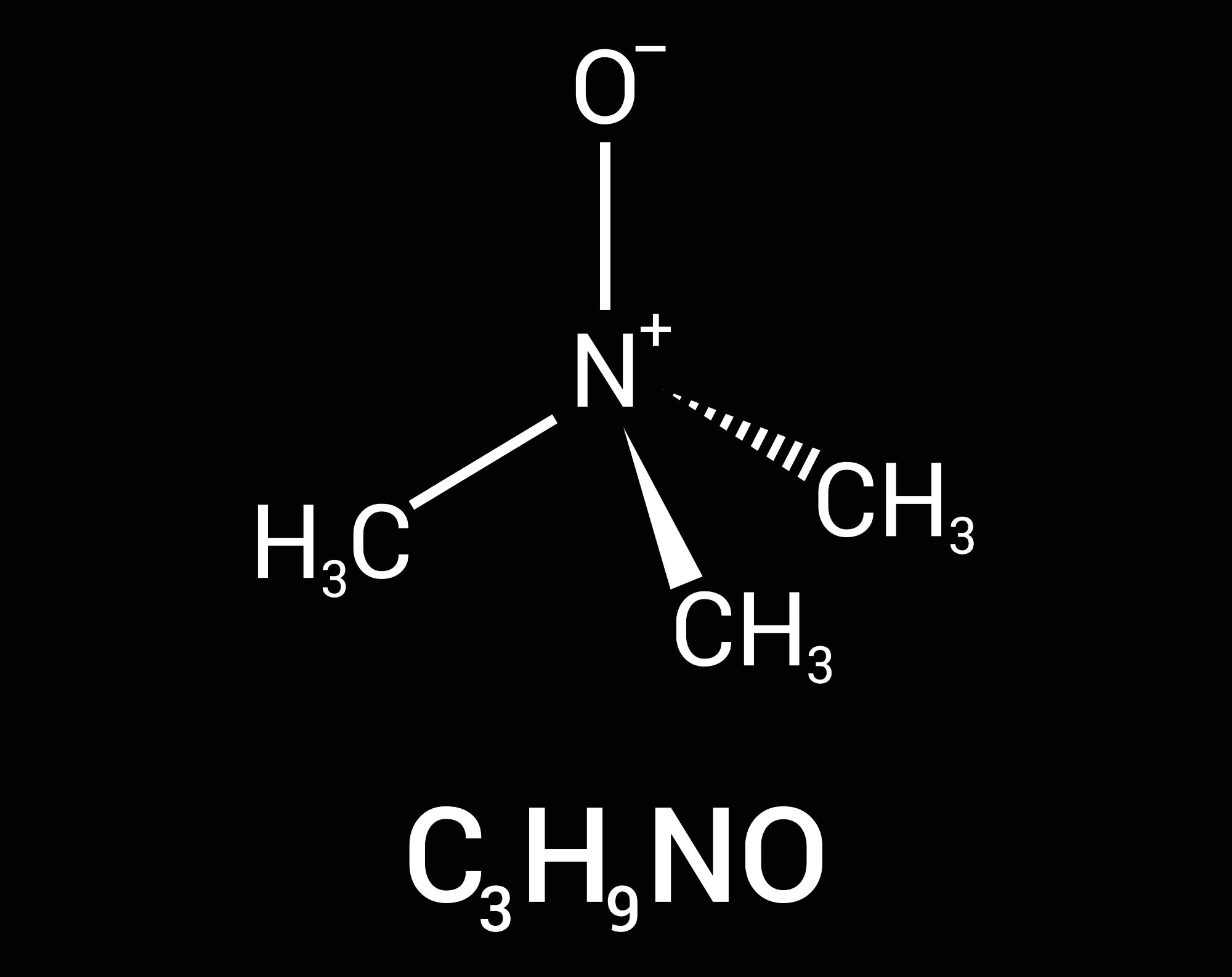
The human gut microbiota plays a crucial role in processing dietary components, especially by producing metabolites that influence health. One such compound, TMAO, forms when gut bacteria break down choline and carnitine, which are found in red meat, eggs, and dairy. These are converted to trimethylamine (TMA), which is then oxidized by the liver enzyme flavin-containing monooxygenase 3 (FMO3) to produce TMAO.
Genetic variation in FMO3, as well as differences in sex hormones and liver function, can influence how efficiently TMA is converted to TMAO, thereby affecting individual susceptibility to disease.
Initially recognized for its osmoprotective role in marine organisms, TMAO has gained attention in human health due to its strong correlation with cardiovascular diseases, atherosclerosis, peripheral artery disease, hypertension, and neurodegenerative disorders, including Alzheimer’s and Parkinson’s diseases.
Elevated TMAO levels have been associated with increased platelet aggregation, endothelial dysfunction, and systemic inflammation—mechanisms that contribute to heart disease and stroke. TMAO also increases intracellular calcium release in platelets, enhancing their reactivity and promoting thrombus formation.
Furthermore, TMAO promotes oxidative stress and inflammation by activating the NLRP3 inflammasome and impairing the SIRT3–SOD2 mitochondrial defense pathway, contributing to both vascular damage and neuroinflammation.
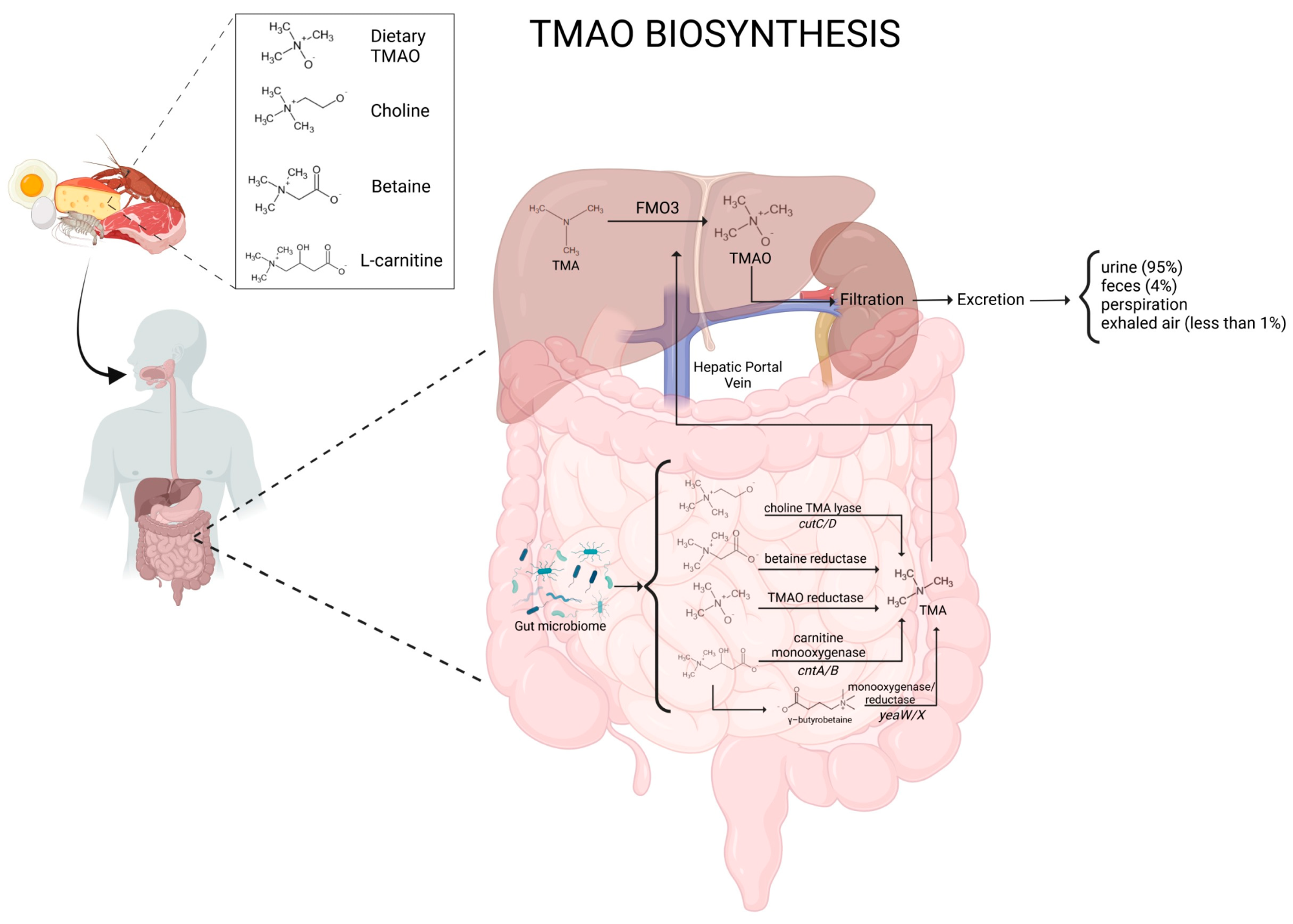 Schematic representation of TMAO biosynthesis and metabolism.
Schematic representation of TMAO biosynthesis and metabolism.
The current study
The review aimed to bridge knowledge gaps by assessing how dietary components, microbiota composition, and potential therapies influence TMAO levels and disease risk.
Researchers analyzed how specific gut bacteria metabolize dietary choline, carnitine, betaine, and other precursors into TMA, which is then transformed into TMAO in the liver. They also evaluated findings from experimental models, clinical trials, and epidemiological studies to clarify TMAO’s involvement in disease.
Dietary patterns were examined by comparing TMAO levels in individuals consuming red meat-heavy diets versus those following plant-based or Mediterranean diets. The team also explored probiotic interventions, nutraceutical compounds, and even pharmaceutical agents that may modulate TMAO synthesis or metabolism.
Major findings
TMAO levels are strongly shaped by both diet and gut microbiota composition. Red meat, eggs, and dairy intake elevate TMAO levels, while plant-based or Mediterranean diets—rich in fiber and polyphenols—are associated with lower levels.
TMAO contributes to cardiovascular disease by impairing nitric oxide production, disrupting lipid and cholesterol metabolism, promoting foam cell formation, and increasing platelet hyperreactivity. It also impairs endothelial progenitor cell function and alters pathways crucial for neovascularization and vascular repair.
Beyond heart disease, the review connects TMAO to cognitive decline, blood–brain barrier dysfunction, and beta-amyloid and tau aggregation—hallmarks of Alzheimer’s. It is also implicated in Parkinson’s disease through mitochondrial dysfunction and chronic inflammation.
Elevated TMAO was associated with poor outcomes in heart failure, increased mortality in peripheral artery disease, and heightened risk of stroke and hypertension.
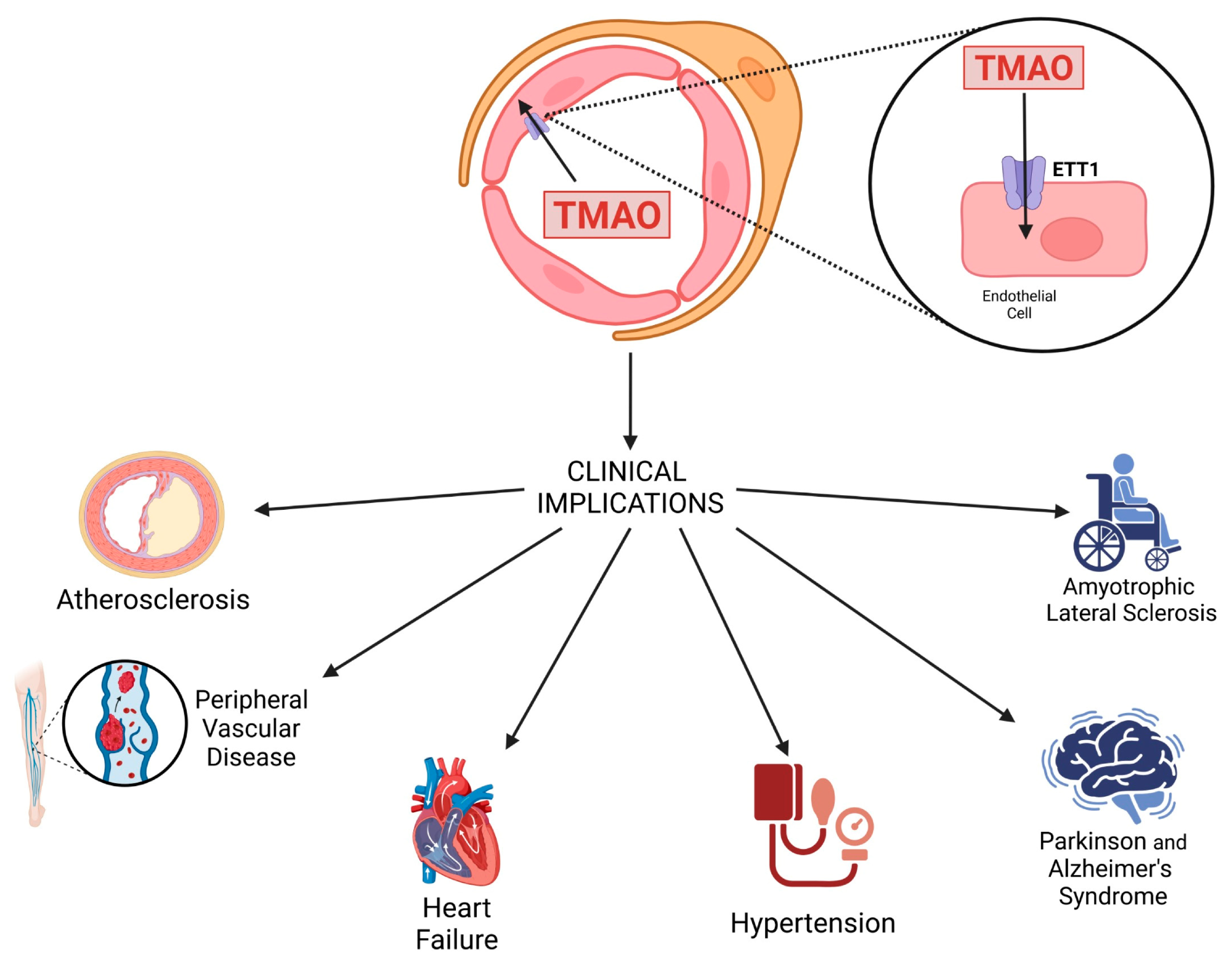 Schematic representation of TMAO transport into endothelial cells via the endothelial TMAO transporter (ETT) and its clinical implications.
Schematic representation of TMAO transport into endothelial cells via the endothelial TMAO transporter (ETT) and its clinical implications.
Therapeutic and lifestyle interventions
Interventions that reduce TMAO include:
- Dietary changes, such as reducing red meat and increasing fiber/polyphenols.
- Probiotics, particularly certain strains of Lactobacillus and Bifidobacterium, which modulate the microbiota to reduce TMA production. However, not all probiotics are effective—formulations like VSL#3 have shown no impact on TMAO levels in trials.
- Nutraceuticals, including resveratrol, quercetin, and the polyphenol-rich supplement Taurisolo®, have shown promising results in reducing TMAO and protecting vascular health.
The review also highlighted the potential role of pharmaceuticals:
- Statins may lower TMAO by modulating gut microbiota and bile acid metabolism.
- ACE inhibitors and loop diuretics indirectly influence TMAO clearance or synthesis via effects on renal excretion and gut flora.
- Monitoring TMAO may enhance cardiovascular risk stratification, particularly in patients with comorbidities.
Despite these advances, the review acknowledged several limitations, including inconsistent probiotic outcomes, variation in individual microbiomes, and a lack of long-term human trials.
Conclusions
TMAO has emerged as a central metabolite linking diet, gut microbiota, host genetics, and disease. The review emphasized that dietary and probiotic strategies, along with personalized approaches based on microbiome and genetic profiling, could offer powerful tools for mitigating TMAO-associated health risks.
Monitoring TMAO levels may also improve the early detection of cardiovascular and neurodegenerative diseases. While research is ongoing, simple changes—such as shifting to a plant-rich diet, using targeted probiotics, or considering nutraceuticals like Taurisolo®—could offer a preventive edge in long-term health.
Journal reference:
- Caradonna, E., Abate, F., Schiano, E., Paparella, F., Ferrara, F., Vanoli, E., Difruscolo, R., Goffredo, V. M., Amato, B., Setacci, C., Setacci, F., & Novellino, E. (2025). Trimethylamine-N-Oxide (TMAO) as a Rising-Star Metabolite: Implications for Human Health. Metabolites, 15(4), 220. DOI: 10.3390/metabo15040220, https://www.mdpi.com/2218-1989/15/4/220