German Innovation and ESGE Innovation of the Year Awards reinforce PENTAX Medical as innovation leader
PENTAX Medical is pleased to announce that the innovation within its new product developments has been recognized by two key awards. The first being the prestigious German Innovation Award 2018 for its ground-breaking DEC™ duodenoscope with a unique disposable design to improve standards of patient hygiene. The second being for PENTAX Medical’s prototype Red Density System, supporting the endoscopic assessment of ulcerative colitis, which has been awarded the ESGE Innovation of the Year Award.
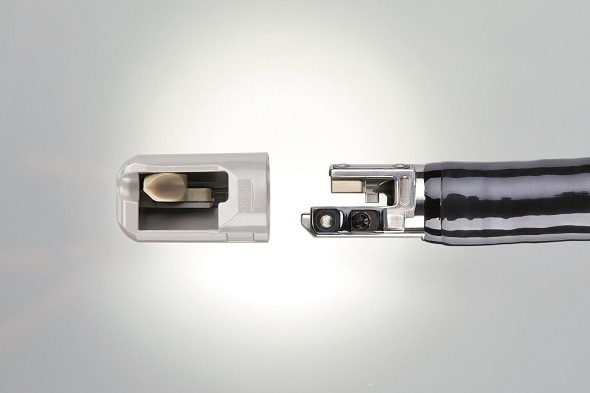
Award winning DEC™ Duodenoscope from PENTAX Medical - aunique duodenoscopewith Disposable Elevator Cap
Reaching the last three top finalists, out of several hundred applicants, PENTAX Medical was the only medical innovator to be nominated for the eminent German Innovation Award 2018 (www.der-deutsche-innovationspreis.de/). For the enhancement to patient safety, enabled by its novel single-use design which allows easier cleaning and reprocessing, the DEC™ Video Duodenoscope ED34‑i10T2 (DEC™ duodenoscope) received second prize in the “Midsized Companies” category at the recent German Innovation Awards Ceremony in Munich.
Once a year the German Ministry of Trade and Industry grants the German Innovation Award to the best innovations born in its economic area. From an R&D perspective this is one of the highest accolades achievable by an R&D group and demonstrates that PENTAX Medical is an innovation leader. The pioneering engineering work undertaken by PENTAX Medical’s R&D team to successfully introduce the simple yet highly effective DEC™ (Disposable Elevator Cap) solution has delivered a world-first combination of advanced cleaning capabilities for infection prevention with high definition image quality.
Through the new DEC™, the R&D team has provided improved medical device hygiene clinical control with HD image quality (HD+) and enhanced procedural performance during diagnostic and therapeutic endoscopic retrograde cholangio-pancreatography (ERCP) procedures. The team responded to the need for single-use products in endoscopy due to increasing incidences of multi-resistant bacterial infections that may be linked to improper cleaning or disinfection of the elevator mechanisms of duodenoscopes. The development of DEC™ was also fully supported by feedback from Key Opinion Leader clinicians participating in PENTAX Medical’s Blackbox Innovations™ global endoscopic R&D program.
Following the launch of DEC™ and its recent accolade as a finalist in the German Innovation Awards 2018, also reinforcing PENTAX Medical’s status as an innovation leader, another new product development has been recognized at ESGE Days 2018. In collaboration with University Hospitals Leuven, Belgium, PENTAX Medical’s prototype Red Density system has been awarded the ESGE Innovation of the Year Award. Clinicians at the Department of Gastroenterology at Leuven undertook an evaluation study of this novel software which calculates the endoscopic inflammation in ulcerative colitis (UC).
In an abstract submitted to the ESGE Days Congress, the study concluded that Red Density provides an operator-independent objective tool that has a stronger correlation with histological inflammation than other available endoscopic scores. This means that it has prognostic potential and could improve therapeutic decisions in UC.
Rainer Burkard, President EMEA, PENTAX Medical commented:
We are immensely proud to receive both of these Awards which highlight our expertise and strength in innovative new product development to successfully meet specific endoscopy needs. With patient safety and hygiene positioned at the forefront of our product design and development programs, our DEC™ duodenoscope provides a breakthrough solution in infection prevention as a unique single-patient use product. Whilst our Red Density system will ultimately enhance therapeutic decision making. We must congratulate our award-winning R&D team for their incisive innovative work delivering two innovation award winning products in rapid succession!”