PENTAX Medical EMEA announces its continued cooperation with SMART Medical to support the distribution and deployment of SMART’s G-EYE® technology integrated with PENTAX Medical’s HD+ systems to the EMEA market. The G-EYE system employs a permanently-integrated balloon at the tip of the conventional endoscope, which is moderately inflated during endoscope withdrawal and enhances colonoscopy detection capabilities by straightening intestinal folds, centralizing the endoscope optics and eliminating bowel slippage. The result is a significantly improved detection of pre-cancerous polyps (adenomas) and substantial clinical benefit.
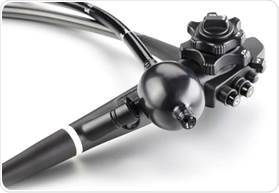
The G-EYE system provides an innovative solution for enhancing adenoma detection rate (ADR), meeting the demand for improved quality in colonoscopy. G-EYE allows increasing ADR and superior stabilization of the endoscope even at difficult locations such as the right colon and tight flexures. Combined with the more controlled withdrawal of the G-EYE system, this provides improved diagnostic and therapeutic capabilities and a faster and more controlled procedure.
Rainer Burkard, President EMEA, PENTAX Medical commented:
Both PENTAX Medical and SMART Medical Systems are committed to the distribution of the G-EYE System to increase ADR across the endoscopy community. At PENTAX Medical, we are pleased to offer the integrated solution of SMART Medical’s G-EYE technology with our innovative HD+ endoscopy systems. We believe it has the capabilities to transform current adenoma detection practices, which is a statement that has been validated through a prospective randomized multi-center study, supported by both companies.
The study included 1,000 patients enrolled across 14 international centers of expertise to compare the detection rate of the G-EYE system with that of standard HD colonoscopy. Intermediate results of 480 patients presented outstanding ADR results, including a 45.6% increase in ADR and a 96.9% increase in the detection of advanced adenomas. The system also received excellent clinical feedback throughout the study.
Prof. Ralf Kiesslich, HELIOS Dr. Schmidt Kliniken, Wiesbaden, Germany who is leading the multi-center study commented:
G-EYE detects significantly more adenomas than standard colonoscopy and reduces colonoscopy miss rates. The increased ADR, advanced adenomas, and adenomas per patient will ultimately impact surveillance intervals of patients undergoing colonoscopy.
Professor Sauid Ishaq, from Russell’s Hall Hospital, Dudley, UK, was one of the clinicians involved in the G-EYE multi-center study. Describing his experience, he said:
The G-EYE endoscope ensures the colon haustral folds are straightened, the endoscope optics are centered within the lumen, bowel slippage is avoided in difficult positions and the overall visibility is improved. In addition, the balloon can help stabilize the endoscope’s tip in difficult conditions. The clinical results of the G-EYE endoscope, together with the accessibility advantages, provide a solid ground for the G-EYE endoscope delivering a high-end solution for day-to-day routine colonoscopy.
In combination with Smart Medical’s NaviAid AB™ through-the-channel balloon device, the G-EYE system also offers on-demand two-balloon endoscopy to meet the market requirement for small intestine procedures. Being a unique, complete solution, the G-EYE system in combination with NaviAid AB delivers additional complementary therapeutic and enteroscopic functions.
Gadi Terliuc, CEO, SMART Medical Systems Ltd. added:
While colonoscopy has long been the technique-of-choice for preventing and diagnosing colorectal cancer, it was well known that many adenomas were unfortunately missed during routine colonoscopy. There was a need for a new product or technique that could reveal missed and hard-to-spot lesions, which is where the G-EYE system excels, providing an almost 50% increase in ADR. Our extended partnership with PENTAX Medical, a market leader in the endoscopy field, can make the G-EYE a widely available solution and a new standard of care in colonoscopy.