Researchers at A*STAR's Singapore Bioimaging Consortium (SBIC) have discovered that branched-chain amino acids (BCAAs) in tumors can be targeted to prevent and treat cancer. Together with collaborators from the United States and National Cancer Centre Singapore (NCCS), they found that some cancers potently suppress the catabolism (breakdown) of BCAAs. This leads to BCAAs accumulating in tumors and activating a known pro-oncogenic pathway called mTOR. Researchers also found that dietary BCAA intake was directly linked to tumor development, suggesting that diets low in BCAAs could limit tumor progression and enhance overall survival.
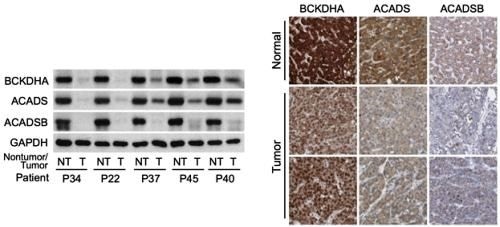
Figure 1- On the left is Western blot analysis on non-tumor (NT) and tumor (T) tissues from HCC patients enrolled at NCCS, showing a sharp decrease in expression of three important BCAA catabolic enzymes (The GAPDH enzyme was used as loading control). On the right are images of normal liver tissues or HCCs stained with antibodies for the three BCAA catabolic enzymes. The images were obtained from the Human Protein Atlas. Normal liver tissues typically have high expression (dark brown), while tumors have weak expression, confirming results from the NCCS cohort.
In recent years, studies have shown that numerous metabolic pathways are altered in cancer cells. However, it has also been reported that many of the same metabolic pathways are altered in normal proliferating cells, suggesting they may simply be related to proliferation, rather than specific to cancer. The researchers designed a project to circumvent this problem by studying not only Hepatocellular Carcinoma (HCC), the predominant form of liver cancer, but also healthy regenerating liver tissues. After identifying BCAA metabolism as a key pathway specific to liver cancer, they proceeded to find similar changes in stomach, colorectal, and kidney cancers, amongst others. Worldwide, cancer is the second leading cause of death, with stomach, colorectal and liver cancers accounting for some of the most common causes of cancer deaths.
SBIC's findings are a result of six years of comprehensive preclinical and clinical research in collaboration with Duke University, the NCCS, and the University of Rhode Island. The research study tapped on SBIC's multi-disciplinary capabilities and involved transcriptomic and metabolomic analyses of human tumors, cancer cell lines, and animal models of liver cancer and regeneration.
Suppression of BCAA Catabolism as a Driver of Liver Cancer Development and Progression
The research team led by Dr. Han Weiping, Deputy Director of SBIC and Head of the Laboratory of Metabolic Medicine (LMM), and Dr. Russell Ericksen, Research Scientist in LMM, first studied gene expression levels in matching tumor and non-tumor liver samples from HCC patients at NCCS, and validated their findings with data gathered from multiple independent HCC cohorts, such as The Cancer Genome Atlas (TCGA). The team found that the catabolism of BCAAs was not only suppressed in tumors when compared to adjacent normal tissue, but the degree of suppression correlated with tumor aggressiveness and disease progression. They also identified three BCAA catabolic enzymes - BCKDHA, ACADS, and ACADSB that were the best predictors of patient survival (Refer to Figure 1 for details).
The changes in BCAA metabolism were confirmed by analyzing metabolite and protein levels, as well as enzyme activity in the matching tumor and non-tumor liver samples. Using a new hyperpolarised magnetic resonance spectroscopy method developed by the SBIC researchers, enzyme activity in the livers of live subjects was monitored in real-time. This non-invasive technique could eventually be used in the clinical setting to screen patients for changes in BCAA catabolism.
By comprehensively analyzing additional cancer subtypes profiled by TCGA, the investigators also found that reduced expression of BCAA catabolic enzymes correlated with tumor development, progression and aggressiveness, as well as patient survival in numerous other cancers. These associations were strongest in cancers of the colon and rectum, stomach, kidney and adrenal cortex. Regardless of cancer subtype, when the TCGA patients were analyzed collectively as a pan-cancer cohort, those with higher tumor expression of BCKDHA, ACADS, and ACADSB lived significantly longer.
Dietary BCAA Intake Linked to Tumor Development and Growth
Given that BCAAs are essential amino acids - meaning they are absorbed from food rather than produced naturally in the body - the researchers also explored how dietary BCAA intake influenced tumor development and growth. In one study, the researchers used a common mouse model of liver cancer, and fed the mice diets with either normal or high levels of BCAAs. After five to eight months, the group of mice fed with high BCAA diets had a potent increase in tumor number and size. Of note, the same diet did not cause any changes in control mice that do not normally develop tumors, suggesting the effects were specific to cancer. Conversely, promoting the catabolism of BCAAs by administering a pharmacological compound, or feeding the mice a low BCAA diet limited tumor growth. These findings signal that BCAA accumulation regulates liver tumor development, and that dietary intervention could influence tumor progression and overall survival (Refer to Figure 2 for details).
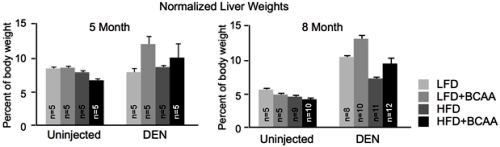
Figure 2: The two charts show the liver masses of mice at five month (left) and eight month (right) time points (normalized to the body weights of the mice). At both time points, mice which were introduced to diethylnitrosaine (DEN) - a compound which generates tumors from within an organism, and fed diets with high levels of BCAAs, had a greater tumor burden, as reflected by increased liver masses. This effect was consistent in diets containing either low or high levels of fat (LFD and HFD, respectively). Notably, the high BCAA diets did not substantially impact the healthy livers of uninjected mice.
Finally, the researchers analyzed the National Health and Nutrition Examination Survey (NHANES) III cohort, which has detailed nutritional and medical information. In agreement with animal studies, this analysis suggested that high dietary intake of BCAA is also associated with a high overall cancer mortality risk in humans (Refer to Figure 3 for details).
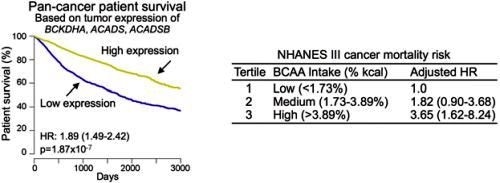
Figure 3: The left chart shows that patients with tumors that have high expression of the genes BCKDHA, ACADS and ACADSB live significantly longer than those with tumors that have low expression. The data was derived from the TCGA pan-cancer cohort of over 7,000 patients. The right chart shows the dietary analysis of the NHANES III cohort. It indicates that humans who have a high dietary intake of BCAA are more likely to develop and die from cancer.
Dr. Han Weiping said:
The current study presents opportunities for the development of prevention and therapeutic intervention strategies to treat several common and deadly cancers. We hope to see that our study eventually leads to new drugs and therapies that benefit patients."
This important study by Dr. Han Weiping's team is key in better understanding the role of the interesting cancer-driving metabolites, BCAAs - not only for liver cancer, but also potentially in other cancers. The finding that dietary BCAAs regulate cellular metabolism uncovers new factors in the cause of liver cancer, and potentially new drug targets."
Associate Professor Toh Han Chong, Senior Consultant Medical Oncologist and Deputy Medical Director (Education), NCCS.
Moving forward, the team will work with NCCS to continue investigating the underlying mechanisms by which BCAAs regulate HCC, and to develop and test the efficacy of drug compounds that may lead to better treatment and outcomes for patients.