 New Africa | Shutterstock
New Africa | Shutterstock
Over the last few decades, we as an industry have shifted away from open surgery towards minimally invasive surgery where possible. This involves surgery through small ports, more commonly referred to as keyhole surgery.
The benefit of this is that it's far less traumatic for patients, reduces recovery time, and lowers the risk of complications and infections, to name a few. One of the downsides is that you no longer have an open surgical site, so you can't directly see what you're doing.
The industry gets around this by putting cameras into the body. During minimally invasive surgery, surgeons will typically insert a camera and a couple of tools into the body through separate ports. This allows them to see what they are doing whilst carrying out the procedure.
Alex: Currently angioscopy is not done using direct visualization as traditionally large fiber bundles are used to pipe light from inside the body back to a camera sensor contained in a large tower. Whilst it is possible to put a small fiber bundle into the body they lack flexibility and are often low resolution. Direct visualization allows surgeons to make diagnostic and treatment decisions based on “line of sight” images in nonstandard cases.
Why is innovation needed in this field?
Simon: One of the most common cardiovascular procedures is removing a blockage in a blood vessel. The problem is that there's no way you can do that as an open surgery - you can't cut into a blood vessel and operate on it. The solution is to do it through a catheter, which is inserted into the blood vessel and moved along the vasculature to the target area.
The challenge is that unlike minimally invasive laparoscopic surgery, you don't have the luxury of space and getting multiple tools and a camera into that area is impossible. Instead, a specialist set of tools are used which are delivered through a catheter.
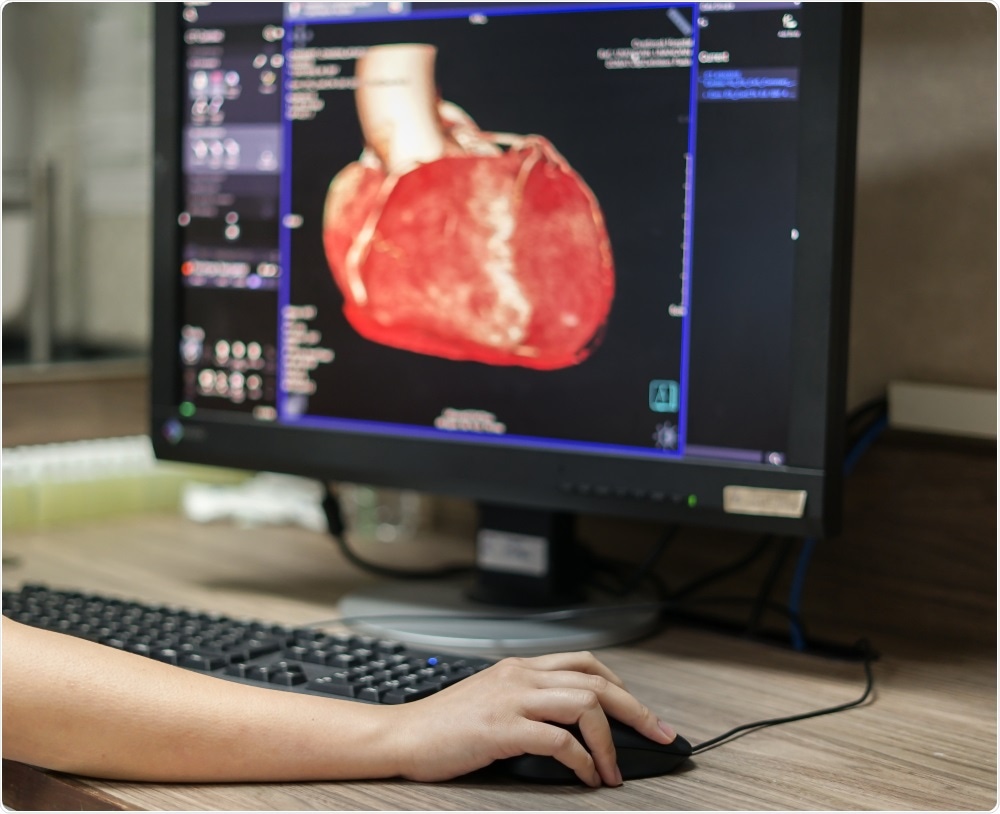
The catheter is inserted into the blood vessel and a tool is run down that catheter to the target site. This gives the surgeon access to the blood vessel but doesn’t allow them to see what they’re doing. Currently, cardiovascular surgeons use external imaging techniques such as fluoroscopy to see what the blood flow looks like, understand where the tool is positioned, and then determine whether they've reached the target site and carry out the procedure.
Performing surgery this way comes with a whole load of associated challenges. Firstly, you have to expose the patient to X-ray radiation, which clinical practice says you want to minimize as much as possible. In addition, it's not real-time imaging; you're taking a series of snapshots to see if you are where you want to be and that's a very disconnected way of seeing what's going on in surgery.
On top of that, the procedural team which performs these surgeries every day is continually being exposed to X-ray radiation. There are lots of protective practices in place, such as wearing lead aprons, standing behind screens etc., but these things get in the way of surgery and aren’t 100% effective.
We therefore need a better, more efficient way of performing intravascular surgery that provides real-time imaging and reduces the need for radiation.
Alex: The thing to clarify is that current surgical procedures for removing occlusions are all safe. Sixty to seventy percent of cases are called ‘standard cases’, which the surgeons have seen thousands of times before and know exactly what's happening.
Intravascular imaging becomes really interesting in non-standard cases, where a surgeon sees something from the outside, puts the catheter in, pokes the occlusion and says, "I don't know what that is." That occurs in a small percentage of cases and having a technology that gives the surgeon direct visualization of the occlusion will massively improve diagnosis and treatment plans.
 pang_oasis | Shutterstock
pang_oasis | Shutterstock
What is Leap and how it revolutionizing cardiovascular surgery?
Simon: Leap is a miniature camera that is small enough to fit inside blood vessels. To give some technical specs, it's about 1.35mm in diameter. However, just putting a camera inside the body isn't too helpful because it's dark in there, so you have to bring some light in as well. That’s why Leap has powerful illumination as well as a camera.
The way that most endoscopes work today is that a set of fiber optics are put into the body, and some of those fiber optics carry light down to what's called the distal tip (the bit of the device that's inside the body). The rest of the fibers carry that light back out of the body, where there's a camera that captures the light.
The problem with this is that the fibers have somewhat limited flexibility and you need to have lenses on the end of the scope, which creates challenges with the size of the scopes as they become quite big.
We've reversed this model and from a camera outside of the body combined with fibers and lenses we have moved to a camera that can be inserted into the body. Now all you need to do is run some wires down the scope.
We've taken a tiny camera and placed it on the tip of the endoscope rather than outside of the body in the surgeon's hand. This is called chip-on-tip technology.
There have been various attempts at chip-on-tip technology, but we set ourselves the challenge of making increasingly miniaturized cameras and seeing how far we can push the size of the endoscope. Once we got down to a scope that was below 2mm, it became apparent that this is something that could be used in cardiovascular applications.
Alex and the team have worked with cardiovascular surgeons in developing the prototype that we have, and the response has been overwhelming from the surgeons that we've shown prototypes to.
There's no surgeon who wouldn't say, "If I can have more information, I would like it." With a camera at this scale, it can get into most major vessels in the body, meaning they have a direct view of that blood vessel in a way that doesn't require them to change the procedure.
Another added benefit is financial. Traditional scopes are very costly and carrying out a laparoscopy today requires a lot of equipment. The scopes themselves are very expensive (several thousand pounds) and therefore must be reused.
Alex: The cost of maintenance and sterilization is in the region of $1500 USD per use for a traditional endoscope, and a traditional scope costs more than $10,000 USD.
Simon: Our technology means that surgery no longer incorporates fibers and all of the processing needed to get a good image, reducing costs. We're not currently producing this in volume, but we estimate that it is entirely feasible it could be produced as a single use disposable scope. This removes the need to worry about sterilization, cross infection, cleaning, and so on.
What other imaging technologies are you currently working on?
Simon: There are a wealth of technologies currently being developed which are aimed at delivering information to surgeons in real-time and seeing if we can start to detect the tissue that we want to operate on.
The classic example that everybody uses, and a huge amount of effort is going into right now, is cancer. Imagine looking at a video of the inside of your patient and the system being able to tell you, "The tumor is here. This is good tissue, this is bad tissue."
Today, surgeons excise what they think to be tumor tissue based on preoperative imaging and their extensive experience, before sending the sample off to the pathology laboratory. The scientists then use immunohistochemical techniques to determine whether the tumor has clean margins.
In many cases, the patient is lying on the operating table while they're doing that analysis. So, the industry is now searching for alternative technologies that can detect the lesion during the surgery, without needing to send a sample to the lab.
There are technologies like fluoroscopy and Raman spectroscopy and a whole bundle of others, many of which use images from cameras, usually with a special light, to try and detect cancer. These are all dependent on having the analytical and visualization techniques in place.
One of the major challenges with small, digital cameras is that the image is limited by the size of the chip. There's a certain level of quality that you need as a surgeon to be able to make use of the image and to do some of the more advanced techniques, you need even higher resolution.
We took this problem to our internal artificial intelligence and machine learning lab to see if we could improve the quality of the image generated by our tiny camera through some clever algorithms. They are now trying to improve the native, low-resolution image using very advanced artificial intelligence algorithms.
Alex: At the moment we are using a 400x400 pixel camera, which gives you a 0.16-megapixel image, but with machine learning, we could take it up to something like between 2-2.5 megapixels.
Where can readers find more information?
Find out more about Leap.
About Simon Karger
 Simon Karger is the Commercial Director of Cambridge Consultants, leading the surgical group.
Simon Karger is the Commercial Director of Cambridge Consultants, leading the surgical group.
His focus is on helping clients to develop breakthrough surgical and implanted devices.
About Alex Joseph

Alex Joseph is a Senior Mechatronics and Systems Engineer at Cambridge Consultants where he primarily works on complex multidisciplinary projects across the Medical Industry.