A new technique to study tissue samples in 3D has revealed that pancreatic cancers can start and grow in two distinct ways, solving a decades-old mystery of how tumors form.
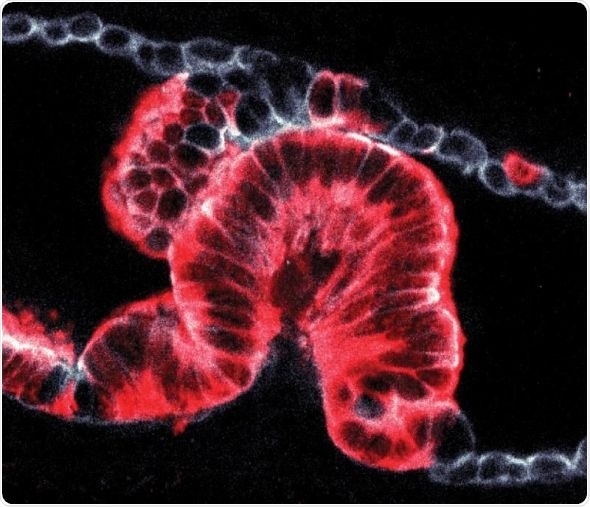
Cell-by-cell image of a tumor (shown in red) growing into a pancreatic duct. Credit Hendrik Massal
The new method could help researchers to get more information from tissue biopsies and may lead to improved treatments for pancreatic cancers. The technique was developed by scientists at the Francis Crick Institute, and their results are published in Nature. The work was supported by the European Research Council and core funding from Cancer Research UK, the Medical Research Council and Wellcome.
The pancreas is a crucial organ that sits behind our stomach and plays a key role in digestion. It relies on a network of ducts linking it to other digestive organs, and the most common pancreatic cancers are found in the ducts. However, until now it has only been possible to see 2D slices of these ductal cancers, which contained an unexplained variety of abnormal shapes.
To investigate the origins of pancreatic cancer, we spent six years developing a new method to analyze cancer biopsies in three dimensions. This technique revealed that cancers develop in the duct walls and either grow inwards or outwards depending on the size of the duct. This explains the mysterious shape differences that we’ve been seeing in 2D slices for decades.”
Dr Hendrik Messal, Francis Crick Institute, co-lead author of the research paper
By analyzing developing cancers in 3D, the team defined two distinct types of cancer formation originating from ductal cells: ‘endophytic’ tumors which grow into the ducts and ‘exophytic’ tumors which grow outwards. To find out what makes cancer cells grow in a particular way, they analyzed detailed 3D images and worked with biophysicists at the Crick who created sophisticated computer models.
“We made a simulation of the ducts, describing individual cell geometry to understand tissue shape,” explains biophysicist Dr Silvanus Alt, co-lead author of the paper. “The model and experimental results both confirmed that cancer grew outwards when the diameter of the duct was less than approximately twenty micrometers, around a fiftieth of a millimeter.”
The work was made possible by an interdisciplinary collaboration between two research groups at the Crick, led by Dr Axel Behrens and Dr Guillaume Salbreux. Axel’s group works on stem cells and pancreatic cancer, while Guillaume focuses on using physics to understand biological processes.
“I think we first started discussing this when we bumped into each other in the bike shed,” says Axel. “It’s amazing what can come out of a chance encounter, we now have a patented technique to see the three-dimensional shapes of cancers and a biophysical understanding of the emergence of tumors. Now that we know pancreatic cancer can develop in these two different ways, we can start looking at whether one is likely to be more aggressive or spread in a different way. Many years from now, this could lead to improved diagnostic or treatment options.”
The team also applied the technique to other organs, and found that cancers in the airways of the lungs and ducts in the liver behave in the same way. This shows that the mechanism the teams discovered is not specific to the pancreas and also applies to other cancers.
“Both the data and our models indicate that the two different mechanisms of tumor growth are purely down to the innate physics of the system,” explains Dr Guillaume Salbreux. “Like most cancers, ductal pancreatic cancer starts with a single defective cell that starts dividing. We found that very quickly, when there are only a few cells, the tumor has already started to grow either inwards or outwards depending on duct diameter. Defining this fundamental process will help us to better understand how cancer grows in many places across the body.”
This technological breakthrough has the potential to unlock many unanswered questions of great importance in how we understand and treat pancreatic cancer. It’s crucial we better grasp how these cancers behave from the earliest stages, to help develop treatments for a disease where survival rates have remained stubbornly low.
Professor Andrew Biankin, Cancer Research UK’s Pancreatic Cancer Expert.