Synopsys are pleased to announce the release of the Synopsys Simpleware™ ScanIP Medical edition. This exciting new version of Simpleware ScanIP comes with FDA 510(k) clearance, and CE and ISO 13485:2016 certification as a medical device.
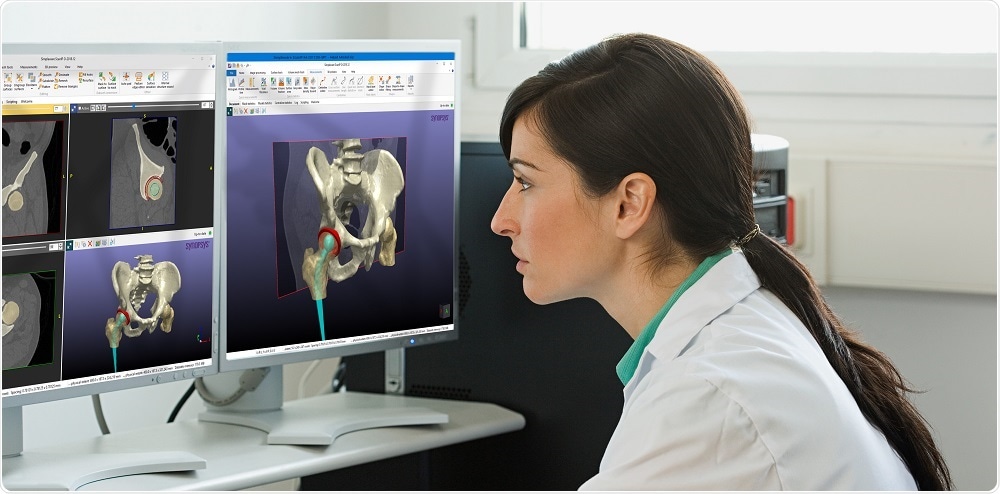
Simpleware ScanIP Medical is the ideal choice for those working with 3D imaging to create medical devices for pre-clinical workflows such as implant design and patient-specific planning.
Developed and maintained to international standards for medical device software, Simpleware ScanIP Medical comes with all the outstanding features of ScanIP, but is specifically intended for medical usage. Process medical scan data (MRI, CT…) and export output files to simulate/evaluate pre-surgical treatment options.
Simpleware ScanIP Medical Workflow Highlights
- Import and anonymize patient DICOM tags from PACS server (DICOM 3.0 compliant)
- Combine CAD and imaging data for patient-specific analysis
- Rapidly segment and process medical images with easy-to-use interface
- Obtain reliable data for complex anatomical analysis using measurement and statistics tools
- Export geometrically accurate models for pre-surgical planning
Key Benefits
- Intuitive, fully supported user interface tested by medical professionals
- Compliant with privacy standards for handling patient data
- Achieve ideal surgical outcomes based on insights from imaging data
- Improve understanding of patient anatomy when designing unique medical devices
- Streamline software resources with complete medical image processing platform for your R&D workstation, radiology department, or other clinical work environments
- Build confidence in surgical decision-making by simplifying time—consuming workflows
For more detailed information on Simpleware ScanIP Medical and its certification, please see our Regulatory Information page.
Those that still want to use Synopsys Simpleware ScanIP for non-clinical medical applications, such as research in the Life Sciences, are recommended to use the core Synopsys Simpleware ScanIP package, which is not intended for clinical use.
More information on the Synopsys Simpleware website
Contact: [email protected]