In recent years, researchers have created mini-organs known as organoids in the culture dish that contain many of the cell types and complex microarchitectures found in human organs, such as the kidney, liver, intestine, and even the brain. However, most organoids grown in vitro lack the vasculature required to provide oxygen and nutrients, remove metabolic waste, and facilitate communication between different cell types that drives their maturation into truly functional tissue building blocks.
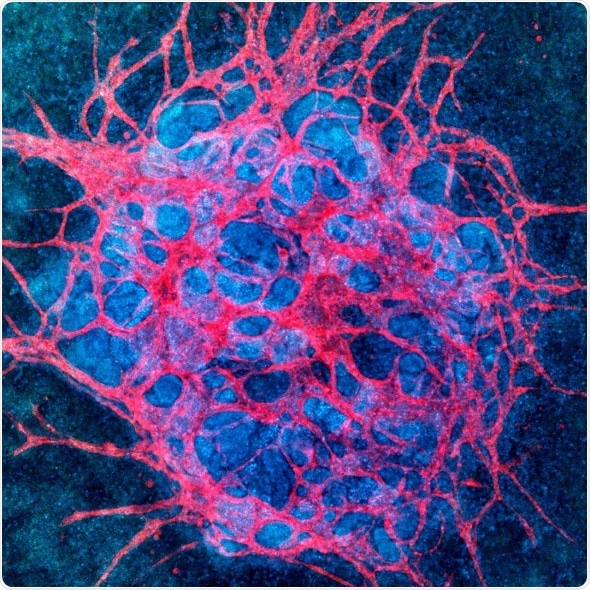
Vascularized kidney organoid: Culture under fluid flow causes endogenous endothelial progenitors in kidney organoids to create more mature vascular networks which pervade the whole organoid and interact with epithelial compartments.
For kidney organoids, this shortcoming prevents researchers from emulating key kidney functions in vitro, including blood filtration, reabsorption, and urine production. Creating robustly vascularized kidney organoids could enable better modeling of kidney diseases, enhance renal drug toxicity testing and, ultimately, lead to new building blocks for renal replacement therapies.
Now, a research team at the Wyss Institute for Biologically Inspired Engineering at Harvard University, Harvard’s John A. Paulson School of Engineering and Applied Sciences (SEAS), Brigham and Women’s Hospital, and the Harvard Stem Cell Institute led by Jennifer Lewis and Ryuji Morizane has developed a powerful new approach as part of the Institute’s new 3D Organ Engineering Initiative. By exposing stem cell-derived organoids to fluidic shear stress, they were able to significantly expand organoid-derived vascular networks, and improve the maturation of kidney compartments in comparison to previous static culture methods. The work is published in Nature Methods.
In 2015, Ryuji Morizane and Joseph Bonventre developed a method that enabled them to derive 3D kidney organoids from human pluripotent stem cells. “While our organoids and those generated in other laboratories contained large numbers of well-organized nephrons and primitive blood vessels, they still lacked pervasive vascular compartments with perfusable lumens,” said co-corresponding author Morizane, M.D., Ph.D., Assistant Professor at Brigham and Women’s Hospital and Harvard Medical School (HMS), and a member of the Harvard Stem Cell Institute.
More recently, researchers around the world have matured kidney organoids by implanting them into animals where they can connect to the host’s vasculature in vivo. “For the first time, our study demonstrates that by exposing growing organoids to fluid flow, a mechanical cue known to play an important role for tissue development in the body, we can greatly enhance their vascularization and maturation in vitro,” said Morizane.
To accomplish this feat, the team used expertise from the Lewis lab that has pioneered strategies to create vascularized human tissues, including 3D kidney-on-chip models, using 3D bioprinting that can be perfused and sustained for long durations. Based on these findings, the researchers hypothesized that fluid flow could also promote the formation of blood vessels from precursor endothelial cells found in growing kidney organoids.
“We determined the right combination of underlying extracellular matrix, media additives, and fluidic shear stress under which human stem-cell derived organoids would flourish when grown in our 3D-printed millifluidic chips,” said Kimberly Homan, Ph.D., who is a first author on the study along with Navin Gupta, M.D. Gupta added that “the vascular networks form close to the epithelial structures that build the glomerular and tubular compartments, and in turn promote epithelial maturation. This integrated process works really like a two-way street.” Homan is a Research Associate in Lewis’ group at the Wyss Institute and SEAS, and Gupta is a Clinical Research Fellow working on Morizane’s team at the Brigham.
The vessels growing on the 3D-printed chips form an interconnected network with open lumens, which can be perfused with fluids as confirmed by directly imaging fluorescent beads moving freely through them. “We were excited to see that these vascularized glomerular and tubular structures develop through some of the same stages that nephrons experience during normal kidney development in vivo,” said Homan.
“This important advance opens up new avenues for accurately testing drug toxicity in vitro in differentiated nephron compartments and modeling kidney diseases, like polycystic kidney disease, that affect specific structures and cell types using patient-derived stem cells as the starting point,” said co-corresponding author Lewis, Sc.D., who is a Core Faculty member of the Wyss Institute and co-leader of its 3D Organ Engineering Initiative. “Our method may pave the way to also vascularize other types of organoids, such as the liver organoids.” Lewis is also the Hansjörg Wyss Professor of Biologically Inspired Engineering at SEAS and a member of the Harvard Stem Cell Institute.
“This study is a great example of the importance of mechanobiology and the potential power of the Wyss Institute’s 3D Organ Engineering Initiative. It provides an important cornerstone for many efforts that aim to create functional human tissues de novo for research, pharmaceutical and tissue regenerative applications,” said Wyss Institute Founding Director Donald Ingber, M.D., Ph.D., who is also the Judah Folkman Professor of Vascular Biology at HMS and the Vascular Biology Program at Boston Children’s Hospital, as well as Professor of Bioengineering at SEAS.
Kidney Organiods: Flow-Enhanced Vascularization and Maturation In Vitro
Kidney Organiods: Flow-Enhanced Vascularization and Maturation In Vitro from Wyss Institute on Vimeo.