This week the African nation of Malawi became one of the first of the planned three to launch a pilot trial for a novel vaccine against malaria. The news and the efforts were lauded by the World Health Organization (WHO).
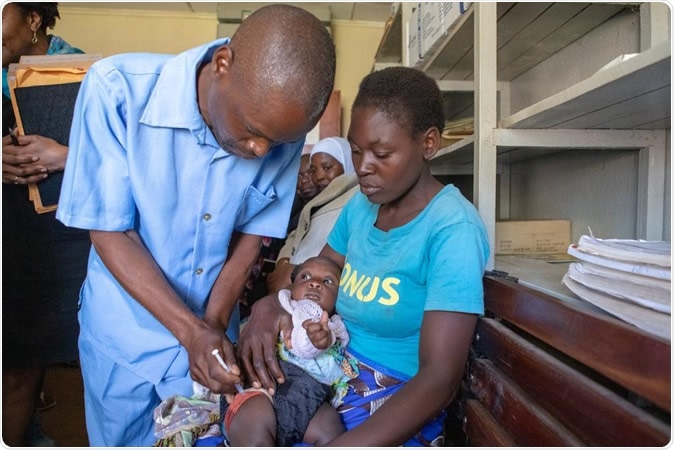
Malaria vaccine trial in Malawi. Image Credit: WHO
According to WHO Director-General Dr Tedros Adhanom Ghebreyesus in a statement, “We have seen tremendous gains from bed nets and other measures to control malaria in the last 15 years, but progress has stalled and even reversed in some areas. We need new solutions to get the malaria response back on track, and this vaccine gives us a promising tool to get there. The malaria vaccine has the potential to save tens of thousands of children’s lives.”
This malaria vaccine took three decades to make say researchers. Until now it remains the only vaccine against malaria. Phase 3 clinical trials with the vaccine have been conducted in Africa between 2009 and 2014. These clinical trials with this vaccine show that this vaccine could prevent 40 percent of malaria cases and 30 percent of fatal severe malaria cases. Dr David Schellenberg, one of the researchers who helped develop the vaccine said in a statement, “There were seven countries participating in a large trial where over 15,000 children participated. [The trial] showed pretty clearly that this vaccine is safe and it is efficacious in terms of its ability to prevent clinical malaria episodes and also severe malaria episodes.”
Dr Matshidiso Moeti, WHO Regional Director for Africa, in a statement said, “Malaria is a constant threat in the African communities where this vaccine will be given. The poorest children suffer the most and are at highest risk of death. We know the power of vaccines to prevent killer diseases and reach children, including those who may not have immediate access to the doctors, nurses and health facilities they need to save them when severe illness comes. This is a day to celebrate as we begin to learn more about what this tool can do to change the trajectory of malaria through childhood vaccination.”
The team leading the pilot trial would roll out the programme and help to generate information and experience with the RTS,S malaria vaccine so that WHO can devise its policy recommendations regarding the vaccine. The utility and efficacy of the vaccine in reducing childhood deaths, safety of the vaccines, if the parents were willing to bring their children for the routine four doses of the vaccine and its general acceptance would all be studied.
WHO officials add, that this new malaria vaccine would be part of the malaria control programme. WHO recommends other major measures for malaria control including insecticide-treated bed nets, use of insecticides indoors and routine testing for malaria in case of fever and its early treatment.
This pilot programme is a collaboration between the WHO and ministries of health in Ghana, Kenya and Malawi along with PATH, a non-profit organization and makers of the vaccine GSK. GSK is donating up to 10 million vaccine doses for this pilot project. This pilot project is receiving financial support from Gavi, the Vaccine Alliance; the Global Fund to Fight AIDS, Tuberculosis and Malaria; and Unitaid. Contributions in kind are being provided by WHO, PATH and GSK.
Steve Davis, President and CEO of PATH said in a statement, “We salute WHO and Malawi for their leadership in realizing this historic milestone and we look forward to the start of vaccination in Ghana, and then Kenya later this year. A vaccine for malaria is among many innovations needed to bring an end to this disease, and we proudly stand with all countries and our many partners in progressing towards a malaria-free world.”
Dr Thomas Breuer, Chief Medical Officer of GSK Vaccines in a statement said, “Delivering the world’s first malaria vaccine will help reduce the burden of one of the most pressing health challenges globally. This novel tool is the result of GSK employees collaborating with their partners, applying the latest in vaccine science to contribute to the fight against malaria. We look forward to seeing the results of the pilot, and in parallel, are working with WHO and PATH to secure the vaccine’s sustained global health impact in the future.”
Dr Seth Berkley, CEO of Gavi in his statement said, “Malaria is still one of the biggest killers of children worldwide, taking the lives of over 200,000 children every year. These pilots will be crucial to determine the part this vaccine could play in reducing the burden this disease continues to place on the world’s poorest countries.”
Similarly Lelio Marmora, Executive Director of Unitaid added, “The malaria vaccine is an exciting innovation that complements the global health community's efforts to end the malaria epidemic. It is also a shining example of the kind of inter-agency coordination that we need. We look forward to learning how the vaccine can be integrated for greatest impact into our work.”
Peter Sands, Executive Director of the Global Fund in his statement added, “To step up the fight against malaria, we need every available tool. If this pilot shows that RTS,S is a cost-effective tool against malaria, it will help us save more children’s lives.”
The pilot programme is slated to reach around 360,000 children annually in the three target nations. The areas covered would be regions where malaria transmission is high and the vaccine would be of greater help say officials. There would be four doses of the vaccine for each child – 3 doses to be administered between 5 and 9 months of age and the fourth dose to be administered at around the age of 2 years.
A PATH white paper on this project says, “Looking ahead The pilot implementation was designed as a six-year program, including preparation, and the evaluations are now expected to be completed by 2023. Its goal is to enable an updated WHO policy recommendation on the possible broader use of RTS,S in African children by generating additional evidence on feasibility, impact, and safety—all in the context of routine use.”