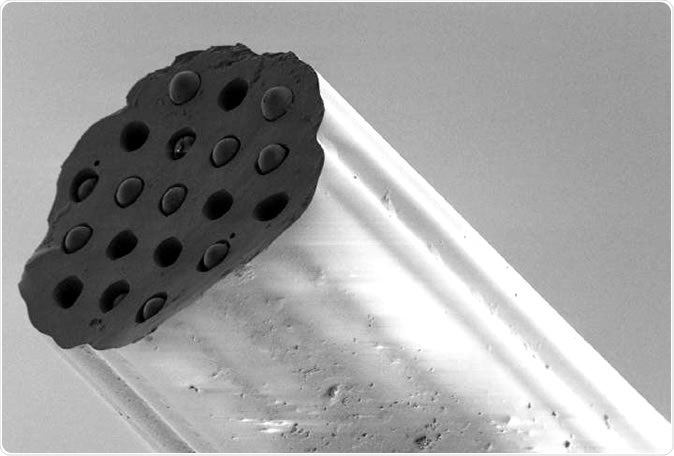
The hair-sized probe can measure key indicators of tissue damage deep in the lung. Image Credit: Michael Tanner, Herriot-Watt University and the University of Edinburgh.
Current knowledge of human physiology comes mostly from animal models. Sensing in humans is far more difficult or impossible because the technology required is invasive, or simply not yet available. The importance of this novel probe lies in its scope to enormously expand in vivo sensing to a host of currently inaccessible areas of the human body, extending the knowledge of body function in both health and disease.
The current study used an ovine lung model which was supplied with oxygen and nutritional fluid through ventilation and perfusion channels. Lung diseases are leading causes of both disability and death today. Despite the progress made, its pathogenesis in conditions like lung trauma or pneumonia is unclear. The new probe could help us understand lung disease and its treatment much better.
A host of optical fiber sensors already exist for pH and oxygen sensing that use sol-gel or polymer coatings to hold the sensing molecules like dyes or nano/microsphere particles to the fiber facet. This type of coupling leads to imprecise deposition, slower response times, and reduced accuracy and range of sensing.
A more recent dual sensing probe used sol-gel or hydrogel coating to deposit both pH and oxygen sensing microspheres on the same optical fiber, but still had relatively slow response times.
The specially designed multicore probe ensures robust sensing, but avoids common issues with fluorescent probes, such as photobleaching and variation of laser pump power.
It has a diameter of 150 microns, with 19 cores each about 10 microns across. Hydrofluoric acid was used to accurately etch pits on the distal surface (facet) that line up with the cores. These pits are irreversibly coupled with 10 micron silica microspheres that covalently bind novel fluorescent pH indicators in ratiometric array and palladium-porphyrin complex based oxygen sensors, at high density.
Fluorescein dye emits fluorescence whose intensity is proportional to the pH, and has been therefore used in a range of pH sensing technologies. However, in the current probe, the saturation loading of the carboxyfluorescein indicator on the microspheres results in a spacing of ~ 5 Angstroms between the dye molecules. This ultra-dense packing gives rise to fluorescent excitation with subsequent energy transfer to neighbors, resulting in a fluorescence response that is inversely proportional to the pH. The emission behavior returns to the expected pattern at standard loading. This unexpected phenomenon, which has never been reported before, greatly boosts the sensor’s performance.
Ratiometric pH sensing is achieved using a FRET pair as fluorophores. Its components are excited by slightly different wavelengths. This causes a more complex, stable and reliable response to pH changes than a single fluorescent response.
The oxygen sensing is by a palladium-porphyrin complex that shows inverse fluorescence as oxygen levels increase. The excitation wavelength was chosen to minimize background fluorescence which could confuse the sensors.
Each core is selectively coupled to and measures fluorescence excited by a single proximal light source. This is responsible for the multiplex nature of the sensing assembly across the multicore fiber.
What advantages does the new probe have?
The covalent pit-microsphere coupling allows almost instantaneous sensing and confers stability to light. The multicore-fiber design allows more sensors to be added to the multiplexed platform as needed. The manufacturing process is also simple, rugged, and reliable, with little effort required for the self-directed microsphere- fiber assembly. The number of fiber cores, the fiber size, and the microsphere size are adaptable to the specific application. The pre-loading deposition of the fluorophore sensors is a repeatable and efficient chemical process which is controllable.
Next, the researchers plan to enclose the fiber sensor assembly in biocompatible materials for testing in various clinical scenarios. This is made simpler by the fact that it can withstand standard sterilization procedures.
Researcher Michael Tanner says: “These new methods, if taken to clinic, will lead to novel insights in disease biology. Our aim now is to expand the number of unique sensors on this miniaturised platform to provide even more information.”
Source:
High fidelity fibre-based physiological sensing deep in tissue, Tushar R. Choudhary, Michael G. Tanner, Alicia Megia-Fernandez, Kerrianne Harrington, Harry A. Wood, Adam Marshall, Patricia Zhu, Sunay V. Chankeshwara, Debaditya Choudhury, Graham Monro, Muhammed Ucuncu, Fei Yu, Rory R. Duncan, Robert R. Thomson, Kevin Dhaliwal & Mark Bradley, Scientific Reports 9, Article number: 7713 (2019), 0.1038/s41598-019-44077-7, https://www.nature.com/articles/s41598-019-44077-7