Oregon State University researchers have come up with nanoclusters that are capable of reaching within tumours that are difficult to reach. The results of this breakthrough in cancer therapeutics were published in a study in the latest issue of the journal ACS Nano. The study is titled, “Biocompatible Nanoclusters with High Heating Efficiency for Systemically Delivered Magnetic Hyperthermia.”
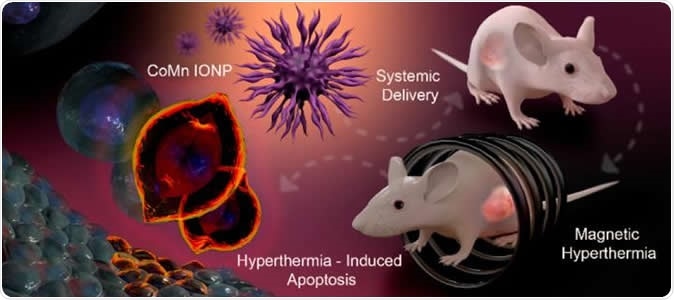
Graphic depiction of the process of using cobalt- and manganese-doped nanoparticles to kill tumors via magnetic hyperthermia. Image Credit: Tetiana Korzun
These nanoclusters are magnetic and are of the size of a billionth of a metre, explain the researchers. They can be easily injected into the body into the cancerous growth and can reach the insides of the tumours easily, they add. Once the nanoclusters have been injected into the tumour, they are subjected to an alternate magnetic field (AMF) from outside, write the researchers. This raises the temperature of the clusters to over 100 degrees Fahrenheit and the heat kills the cancer cells from inside.
The team of researchers explain that the hurdle they faced until now was to find the right nanoparticles that could be able to be injected both directly into the tumour as well as via the intravenous route. Both ways they should be able to reach the tumour mass. Once they accumulate within the tumours, the AMF could be used to kill the cancer cells from within. For ovarian cancers and prostate cancer, these nanoparticles have been used intravenously, they write. The team of researchers included OSU electrical engineering professor Pallavi Dhagat, who led the efforts in developing the naoclusters.
Lead authors Olena Taratula and Oleh Taratula of the OSU College of Pharmacy along with their colleagues developed nanoclusters. These clusters are multiple nanoparticles that have a better heating efficiency. Each of the hexagon-shaped iron oxide nanoparticles are coated with cobalt and manganese and are carried into the body using “biocompatible PEG–PCL (poly(ethylene glycol)-b-poly(ε-caprolactone))-based nanocarriers”, the authors explained.
Taratula, associate professor of pharmaceutical sciences in a statement said, “There had been many attempts to develop nanoparticles that could be administered systemically in safe doses and still allow for hot enough temperatures inside the tumor. Our new nanoplatform is a milestone for treating difficult-to-access tumors with magnetic hyperthermia. This is a proof of concept, and the nanoclusters could potentially be optimized for even greater heating efficiency.” She said that this process of magnetic hyperthermia could now be used for cancer treatment. She said, “It's already been shown that magnetic hyperthermia at moderate temperatures increases the susceptibility of cancer cells to chemotherapy, radiation and immunotherapy.”
For the study the team used mouse models in the labs to show that after the animals had been given IV nanocluster injections, they could be treated with magnetic hyperthermia. In the test animals, ovarian cancer was implanted under the skin for the experiment. Taratula explained, “To advance this technology, future studies need to use orthotopic animal models - models where deep-seated tumors are studied in the location they would actually occur in the body. In addition, to minimize the heating of healthy tissue, current AMF systems need to be optimized, or new ones developed.”
The authors of the study wrote in conclusion, “this nanoplatform is a milestone in the development of systemically delivered magnetic hyperthermia for the treatment of cancer tumors that are difficult to access for intratumoral injection.”
The study was supported by the National Institutes of Health, the OSU College of Pharmacy and Najran University of Saudi Arabia.
Related research
Authors Angl Apostolova from the University of Architecture, Civil Engineering and Geodesy in Sofia, Bulgaria and colleagues this year (April 2019) published their work on magnetic hyperthermia using nanoclusters. Their study was published in the The European Physical Journal B. The study was titled, “Specific absorption rate in Zn-doted ferrites for self-controlled magnetic hyperthermia.”
This study showed that the absorption rate of the heat produced by the nanoclusters depends on the composition of the magnetic material as well as its diameter. The team explained that AMF would be used to activate their magnetic particles. Further they assure that these nanoparticles would be absorbed only by the tumour tissues and not by the healthy tissues. To ensure this the team used iron oxide nanoparticles called ferrite, to which they added small amounts of copper, nickel, manganese or cobalt atoms. This is called dopping.
They tested the efficacy of the nanoparticles then on mice as well as cell cultures and found that diameter of the particles as well as its composition affected the efficiency of the particles to generate magnetic hyperthermia. They explain that For cobalt dopping the maximum absorption is seen with maximum of 14 nanometres size and for copper dopping maximum absorption is seen with maximum of 16 nanometres size.
Journal references:
- Biocompatible Nanoclusters with High Heating Efficiency for Systemically Delivered Magnetic Hyperthermia, Hassan A. Albarqi, Leon H. Wong, Canan Schumann, Fahad Y. Sabei, Tetiana Korzun, Xiaoning Li, Mikkel N. Hansen, Pallavi Dhagat, Abraham S. Moses, Olena Taratula, and Oleh Taratula, ACS Nano 2019 13 (6), 6383-6395, DOI: 10.1021/acsnano.8b06542 - https://pubs.acs.org/doi/10.1021/acsnano.8b06542
- Specific absorption rate in Zn-doted ferrites for self-controlled magnetic hyperthermia, Apostolov, A., Apostolova, I. & Wesselinowa, J. Eur. Phys. J. B (2019) 92: 58. https://doi.org/10.1140/epjb/e2019-90567-2