It is a well known fact that the sun’s ultraviolet rays can damage the skin. Now, a new study sheds light on what happens after DNA damage occurs, and how the body initiates repair.
Ultraviolet (UV) light from the sun is a common carcinogen that can damage the DNA (deoxyribonucleic acid) in the body. DNA is important because it carries genetic information and blueprints for cellular functions. When the body fails to repair the DNA, damage induced by UV light, it can lead to mutations, and eventually, cancer.
UV light poses a threat on genome integrity by triggering DNA damage, also dubbed as intra-strand crosslink damage. There are two major lesions, including the cyclobutane pyrimidine dimer (CPD), which is about 70 percent of the damage and 6-4 photoproduct (6-4PP), which constitutes 30 percent.
Dr. Jung-Hyun Min's team's breakthrough article provides a better understanding of the dynamic process by which DNA damage is recognized and repaired. (Robert Rogers/Baylor University)
Cellular DNA repair system (NER)
What is the cellular DNA repair system (NER)? Nucleotide excision repair (NER) genes are responsible for the repair of DNA damage brought about by exogenous agents, such as UV light, chemicals, and ionizing radiation. The NER proteins recognize DNA damage, remove the error, and repair the DNA strand.
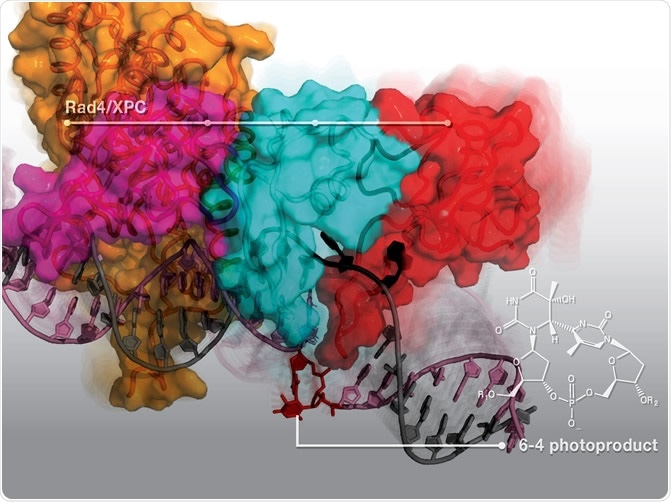
(Credit: Nucleic Acid Research) Rad4/XPC protein dynamically engages with the DNA duplex to 'open' and recognize the 6–4 photoproduct (6–4PP), a major DNA lesion induced by sunlight. Here is presented a new structure that shows how the 6–4PP is completely flipped out of the DNA double helix by Rad4/XPC protein that initiates nucleotide excision repair.
The NER works faster for 6-4PP lesions than on CPD lesions, since the DNA damage-sensing protein known as Rad4/XPC that stimulates NER is more effective at distinguishing 6-4PP lesions. When the Rad4/XPC binds with the lesion, the NER pathway can remove it more efficiently.
Though NER works across all organisms, it remained unclear how the DMA damage-sensing protein distinguishes the lesions and the difference in the efficiency of recognizing them.
To land to the findings of the study, the team of researchers determined a 3D structure of Rad4 protein that is bound to a DNA substrate that contains a 6-4PP lesion. They utilized X-ray crystallography and from there, they saw that the protein flips outward the parts of the DNA that contains the 6-4PP. Hence, it opens the DNA double helix, followed by untwisting and bending the DNA strands.
They also found that the proteins bound not on the damaged parts of the DNA, but instead, on the healthy parts opposite to the lesion. They then computationally stimulated the process wherein the Rad4 may bind with the DNA containing either CPD or 6-4PP. The simulation revealed that the protein readily acts on the 6-4PP to untwist, bend, and partly open the DNA at the site of the lesion. On the other hand, the CPD-containing DNA resisted the untwisting and bending that was readily occurring in 6-4PP.
The researchers put together a 3D molecular trajectory that shows the important steps that occur during the opening of the DNA.
“The hallmark of NER is that it repairs a very broad range of DNA damage. That is quite important in terms of how our genomes are protected from environmentally caused DNA damage,” Dr. Jung-Hyun Min, an associate professor of chemistry and biochemistry in Baylor's College of Arts & Sciences, said in a statement.
"While it has been known for many decades that this Rad4/XPC protein can recognize 6-4PP very efficiently, there has been no structure to show how it really binds to the lesion and why the recognition is so efficient compared with lesions such as CPD. Basically, our study nicely fills this missing gap and details what that mechanism must be,” she added.
The researchers believe that the mechanics of NER could give benefits more than just understanding UV-induced DNA damage, but also the ability of NER to repair such damage. NER is very important to repair damaged DNA strands most commonly caused by cigarette smoke, some chemotherapy drugs, and even industrial pollutants.
"We hope the knowledge we uncover can be helpful in solving major problems in human health," Min said. "This is how we imagine we can help — by understanding how things work with full 3-D structural detail,” Dr. Min added.
What is skin cancer?
One of the most common cancers caused by UV-light exposure is skin cancer.
Skin cancer, which involves the abnormal growth of skin cells, often develop because of the skin’s sun exposure. However, skin cancer can also develop in areas that are not ordinarily exposed to the sun.
The three most common type of skin cancer includes, melanoma, basal cell carcinoma, and squamous cell carcinoma. Melanoma is rare but the most aggressive type of skin cancer. It is more likely to invade surrounding tissues and can spread to the other parts of the body. Melanoma is also the deadliest of all skin cancers.
Skin cancer lesions, like melanoma, can appear and develop anywhere in the body The most common sites, however, are those exposed to the sun, such as the legs, arms, face, and back. But, it can also appear in the soles of the feet, fingernail beds, and the palms of the hands. The most common signs and symptoms include the development of new pigmented or unusual-looking lesion on the skin and a change in an existing mole. To reduce the risk of skin cancer, limiting exposure to ultraviolet radiation or avoiding it is recommended.
Journal reference:
Debamita Paul, Hong Mu, Hong Zhao, Ouathek Ouerfelli, Philip D Jeffrey, Suse Broyde, Jung-Hyun Min, Structure and mechanism of pyrimidine–pyrimidone (6-4) photoproduct recognition by the Rad4/XPC nucleotide excision repair complex, Nucleic Acids Research, Volume 47, Issue 12, 09 July 2019, Pages 6015–6028, https://doi.org/10.1093/nar/gkz359, https://academic.oup.com/nar/article/47/12/6015/5490814