The researchers Josep M. Jornet, Michal K. Stachowiak, Yongho Bae and Ewa K. Stachowiak worked on their complex study to alter genome functions from outside the body and hope that this could mean more effective and feasible gene therapy for cancers and psychiatric conditions such as schizophrenia.
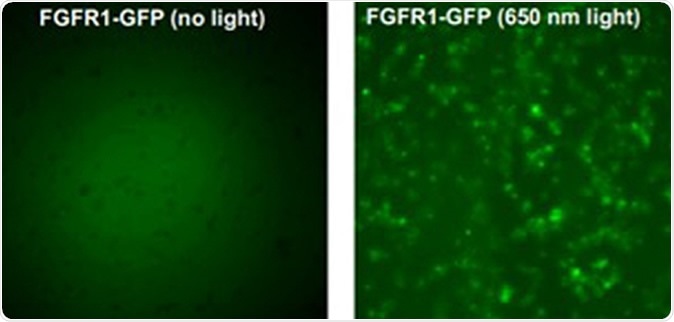
The left image above shows the gene FGFR1 in its natural state. The right image shows the gene when exposed to laser light, which causes the gene to activiate and deactivate. Credit: University at Buffalo.
The researchers explain that their study belongs to the subgenre of science called “optogenomics”. In this technology the team used laser light and nanotechnology to control and alter the genes. Author Josep M. Jornet, PhD, associate professor in the Department of Electrical Engineering in the UB School of Engineering and Applied Sciences, in a statement said, “The potential of optogenomic interfaces is enormous. It could drastically reduce the need for medicinal drugs and other therapies for certain illnesses. It could also change how humans interact with machines.”
The team explains that the gene FGFR1 denotes “Fibroblast Growth Factor Receptor 1”. This gene again is in control of over 4,500 other genes which means around 20 percent of the whole human genome as understood from the Human Genome Project, explained co-author Michal K. Stachowiak. Stachowiak, professor in the Department of Pathology and Anatomical Sciences in the Jacobs School of Medicine and Biomedical Sciences at UB said, “In some respects, it’s like a boss gene. By controlling FGFR1, one can theoretically prevent widespread gene dysregulations in schizophrenia or in breast cancer and other types of cancer.”
The researchers explain that over the last two decades they and others in the field have been attempting to combine optics and genetics. In this they have used light to control how the cells interact with each other and add that this technique may not be useful to correct the genetic malfunctions at present.
In this new study the team used tiny photonic brain implants and used them to control and regulate the functions of FGFR1 – the “boss gene”. These tiny particles are actually miniature wireless devices with nano-lasers and nano-antennas and soon-to-be-added nano-detectors. These photonic implants were inserted into the lab-grown brain tissues. These brain tissues were created from stem cells or pluripotent cells and are called “3-D organoids (minibrains)”. The tissues were then triggered with blue, red and far-red laser beams to activate them. These lights help the researchers to activate and deactivate the FGFR1 gene and thus affect its control over other cellular functions. This was called “gene hacking”. Stachowiak said that this wireless hacking of the genes could be used some day to correct genetic abnormalities.
The team wrote that there have been recent developments in terms of “miniature light sources”. They wrote, “a prominent class of integrated nanolasers is based on microring resonators, i.e., ring-shaped resonant cavities with a footprint on the order of a few micrometers.” They added that these could have potential in “on-chip integrated photonic applications.” The team used technologies called the “overlay lithography technique” to overcome the challenges associated with the use of these microdevices. They wrote that to enhance the activities of their nanolasers, they used “plasmonic nanoantennas”. These would control the radiation and optical signals.
The team wrote, “Three stages of optogenomic interfaces are described, ranging from already experimentally validated interfaces activating broad cellular responses and expressing individual genes to more advanced interfaces able to regulate and correct DNA topology, chromatin structure, and cellular development.”
The team is planning on proving their theories and hypotheses on live animals and say that it may be a long way before wireless genetic alteration is a reality on humans. They wrote, “We acknowledge that this research is still at its fundamental stage, but its potential broader impact on our society motivates and encourages a jointly coordinated effort from the bioscientific and nanoengineering communities.”
Their study was supported by the U.S. National Science Foundation.
The boss gene
The boss gene or the FGFR1 codes for a protein that is part of the fibroblast growth factor receptor (FGFR) family. Throughout evolution the features of this protein has changed very little.
A typical FGFR protein has, “an extracellular region, composed of three immunoglobulin-like domains, a single hydrophobic membrane-spanning segment and a cytoplasmic tyrosine kinase domain.” The portion outside the cells interacts with “fibroblast growth factors” and this helps in regulating several functions of the cells affecting growth and development.
If there is a mutation of the FGFR1 gene there are disease conditions such as “Pfeiffer syndrome, Jackson-Weiss syndrome, Antley-Bixler syndrome, osteoglophonic dysplasia, and autosomal dominant Kallmann syndrome 2,” etc. Alteration of the gene is also associated with “stem cell myeloproliferative disorder and stem cell leukemia lymphoma syndrome.”
https://www.ncbi.nlm.nih.gov/gene/2260
Journal reference:
J. M. Jornet et al., "Optogenomic Interfaces: Bridging Biological Networks With the Electronic Digital World," in Proceedings of the IEEE. doi: 10.1109/JPROC.2019.2916055, http://ieeexplore.ieee.org/stamp/stamp.jsp?tp=&arnumber=8734762&isnumber=4357935